NCERT Exercise Solutions – Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
7.1 Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Ans- the properties of group 15 elements in terms of their atomic size, oxidation state, electronic configuration, and ionisation enthalpy,
These electronegativity include:
(i) Electronic configuration: All the elements of group 15 have 5 electrons encased in their valence shell. These components feature the standard electrical ns2np3 setup
(ii) Oxidation state: Elements from group 15 typically display 3.
oxidation states +3 and +5. the propensity to display a 3 oxidation state due to an increase in the size of the drops as we proceed down the group. The metallic quality and the atom. The stability of the inert pair effect results in As we progress along the group, there are +5 state declines and +3 state gains.
(iii) Electronegativity and ionisation energy: Moving down a group causes a decrease in initial ionisation. The reason for this is the increase in atomic size. Due to an increase in size as we progress down a group, electronegativity drops.
(iv) Atomic size: Atomic size grows as you descend a group. An increase in the number of shells is thought to be the cause of this increase in atomic size.
7.2 Why does the reactivity of nitrogen differ from phosphorus?
Ans- In the form of the diatomic molecule (N2), molecular nitrogen is made up of two nitrogen atoms that are joined by a triple bond (NN). At ambient temperatures, it is a gas. Due of the size of phosphorus, multiple bonding is not conceivable. It exists as a solid known as a P4 molecule, which consists of just one covalent bond between each P atom. Comparatively speaking to molecular phosphorus, molecular nitrogen is far less reactive due to the higher bond dissociation enthalpy (946 kJ mol-1) of the NN bond.
7.3 Discuss the trends in chemical reactivity of group 15 elements.
Ans- Trends in group – 15’s chemical composition generally,
(i) Hydrogen reactivity: Group 15 elements react with hydrogen to generate hydrides of type EH3, where E is one of N, P, As, Sb, or Bi. Moving from NH3 to BiH3 causes the stability of hydrides to decline.
(ii) Reactivity to oxygen: Elements in group 15 can react with oxygen to generate two different types of oxides, E2O3 and E2O5, depending on whether E is N, P, As, Sb, or Bi. More acidic than the other oxide is the one whose element is in a greater oxidation state. Moving down a group, however, results in less acidity.
(iii) Reactivity towards halogens: The group 15 elements react with halogens to form two series of salts: EX3 and EX5. However, nitrogen does not form NX5 as it lacks the d-orbital. All trihalides (except NX3) are stable.
(iv) Reactivity towards metals: The group 15 elements react with metals to form binary compounds in which metals exhibit -3 oxidation states.
7.4 Why does NH3 form hydrogen bond but PH3 does not?
Ans- The electronegativity of nitrogen, which is substantially higher than that of hydrogen, is 3.0. (2.1). Since the N–H bond is extremely polar as a result, NH3 experiences intermolecular H–bonding.
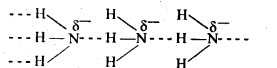
7.5 How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Ans- When an aqueous solution of ammonium chloride (NH4Cl) is heated with sodium nitrite (NaNO2), ammonium nitrite (NH4NO2) is obtained, which is unstable. This ammonium nitrite further breaks down into nitrogen and water. NH4Cl(aq) + NaNO2(aq) → NH4NO2 + NaCl(aq) NH4NO2 → N2(g) + H2O(l) Minute amounts of NO and HNO3 are also produced in the reaction. These impurities can be removed by passing nitrogen gas through aqueous sulphuric acid containing potassium dichromate.
7.6 How is ammonia manufactured industrially?
Ans- Haber’s technique is used extensively in industrial ammonia production. The Haber’s process creates ammonia by mixing airborne nitrogen gas in a 1 to 3 ratio with hydrogen generated from natural gas (methane).
N2(g) + 3H2(g) ⇌ 2NH3(g)
The reaction is reversible, and the production of ammonia is exothermic in nature.
7.7 Illustrate how copper metal can give different products on reaction with HNO3.
Ans- Concentrated nitric acid is a strong oxidizing agent. It is used for oxidizing most metals. The products of oxidation depend on the concentration of the acid, temperature, and also on the material undergoing oxidation.
The reaction of copper with dilute and concentrated HNO3 is as shown below:
3Cu + 8HNO3(dil. ) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3(conc. ) → Cu(NO3)2 + 2NO2 + 2H2O
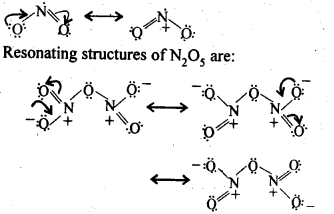
7.8 Give the resonating structures of NO2 and N2O5.
Ans–
7.9 The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? [Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s–p bonding between hydrogen and other elements of the group].
Ans– Hydride NH3 PH3 AsH3 SbH3
H-M-H angle 107° 92° 91° 90°
The electronegativity of the core atom can be used to explain the aforementioned trend in the H-M-H bond angle. There is a lot of electron density surrounding nitrogen because it has a strong electronegative charge. As a result, the electron pairs around nitrogen are more strongly attracted to one another, increasing the bond angle. We are aware that as one descends a group, electronegativity drops. As a result, the H-M-H bond angle decreases due to the diminishing repulsive interactions between the electron pairs.
7.10 Why does R3P = O exist but R3N = O does not (R = alkyl group)?
Ans- In order to increase its octet, nitrogen lacks a d-orbital. Its coordination number is therefore limited to four. However, because phosphorus has a free d-orbital, it can expand its octet and create R3P = O. R3P = O therefore exists, whereas R3N = O does not.
7.11 Explain why NH3 is basic while BiH3 is only feebly basic.
Ans- Since N and Bi both have a single pair of electrons on their core atoms in NH3 and BiH3, they ought to act as Lewis bases. Yet NH3 is a lot more fundamental than BiH3. The electron density on N is significantly higher than that on Bi because N has a substantially lower atomic size than Bi. In contrast to the inclination of Bi in BiH3, N has a far higher tendency to donate its single pair of electrons in NH3. Thus, NH3 is more fundamental than BiH3.
7.12 Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Ans- Due to its small size, nitrogen tends to create many p-bonds with itself. N2, a very stable diatomic molecule made of nitrogen, is the result. The propensity to develop p-p bonds declines as one moves down a group (because of the large size of heavier elements). Consequently, phosphorus occurs in the P4 form, just like other heavier metals.
7.13 Write main differences between the properties of white phosphorus and red phosphorus.
Ans-
| White phosphorus | Red Phosphorus |
|---|---|
| It is a soft and waxy solid. It possesses a garlic smell. | It is a hard and crystalline solid, without any smell. |
| It is poisonous. | It is non-poisonous. |
| It is insoluble in water but soluble in carbon disulphide. | It is insoluble in both water and carbon disulphide. |
| It undergoes spontaneous combustion in air. | It is relatively less reactive. |
| In both solid and vapour states, it exists as a P4 molecule. | It exists as a chain of tetrahedral P4 units. |
7.14 Why does nitrogen show catenation properties less than phosphorus?
Ans- N has an electronic valence shell structure of 2s22p3. The two nitrogen atoms share three electron pairs in the valence p-subshell and form a triple bond (N=N) to complete the octet. Because of this, molecular nitrogen is a separate diatomic species that cannot be linked together or catalysed by several nitrogen atoms. However, because of the element’s relatively high atomic size, multiple bonding is not possible in the case of phosphorus. White phosphorus contains the tetra-atomic molecule (P4) of molecular phosphorus. These tetrahedrons are further connected by covalent bonds to create the polymeric red variant. As a result, nitrogen catenation is lower than phosphorus catenation.
7.15 Give the disproportionation reaction of H3PO3.
Ans- On heating, H3 P04 undergoes self – oxidation-reduction, i.e: disproportionation to form PH3.
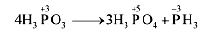
7.16 Can PCl5 act as an oxidising as well as a reducing agent? Justify.
Ans- The sole function of phosphorous pentachloride is as an oxidising agent. Because of the phosphorus’s +5 oxidation state in PCl5, which prevents it from rising above that value, the compound cannot operate as a reducing agent. However, it may readily act as an oxidising agent because it can lower its oxidation state from +5 to +3.
7.17 Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Ans- (i)Electronic configuration:
O (At. no. = 8) = [He] 2s2 2p4
S (At. no. = 16) = [Ne] 3s2 3p4
Se (At. no. = 34) = [Ar] 3d10 4s2 4p4
Te (At. no. = 52) = [Kr] 4d10 5s2 5p4 ,
Po (At. no. = 84) = [Xe] 4f14 5d10 6s2 6p4 ,
Thus, all these elements have the same ns2 np4 (n = 2 to 6) valence shell electronic configuration, hence are justified to be placed in group 16 of the Periodic Table.
(ii) Oxidation state: These elements should exhibit an oxidation state of -2 because they have six valence electrons (ns2 and np4). However, due to its strong electronegativity, oxygen is the sole element that primarily exhibits the oxidation state of 2. Additionally, it displays the oxidation states of -1 (H2O2), 0 (O2), and +2 (O3) (OF2). Moving down a group, however, results in a decrease in the stability of the -2 oxidation state due to a drop in the electronegativity of the elements. Because d-orbitals are available, the heavier members of the group exhibit an oxidation state of +2, +4, and +6.
(iii) Formation of hydrides: Chalcogens form hydrides of formula H2E, where E = O, S, Se, Te, Po. Elements oxygen and sulphur also form hydrides of type H2E2.
7.18 Why is dioxygen a gas but sulphur a solid?
Ans- Compared to sulphur, oxygen is smaller in size. It may effectively form p-p bonds and the O2(O==O) molecule because of its reduced size. Additionally, oxygen exists as a gas because of the weak van der Wall intermolecular interactions. The M2 molecule does not form in the case of sulphur, which instead exists as a puckered structure bound by powerful covalent connections. It is therefore a solid.
7.19 Knowing the electron gain enthalpy values for O → O– and O → O2– as –141 and 702 kJ mol–1 respectively, how can you account for the formation of a large number of oxides having O2– species and not O– ? (Hint: Consider lattice energy factor in the formation of compounds).
Ans- The lattice energy of an ionic molecule determines its stability. The lattice energy of a compound directly relates to the charge that an ion is carrying, and the higher the lattice energy, the more stable the compound will be. When a metal reacts with oxygen, the oxide involving the O2 ion has a substantially higher lattice energy than the oxide involving the O ion. As a result, oxides with O2 ions are more stable than oxides with O. Consequently, the creation of O2 is preferable than that of O.
7.20 Which aerosols deplete ozone?
Ans- Aerosols like freons or chlorofluorocarbons (CFCs) hasten the ozone layer’s depletion. When exposed to ultraviolet radiation, CFC molecules decompose into chlorine-free radicals, which react with ozone to produce oxygen.
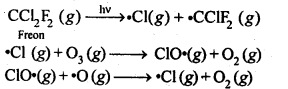
7.21 Describe the manufacture of H2SO4 by contact process?
Ans- Sulphuric acid is manufactured by the contact process. It involves the following steps:
- Sulphur or sulphide ores are burnt in air to form SO2.
- By a reaction with oxygen, SO2 is converted into SO3 in the presence of V2O5 as a catalyst.
- SO3 produced is absorbed on H2SO4 to give H2S2O7 (oleum). This oleum is then diluted to obtain H2SO4 of the desired concentration. In practice, the plant is operated at 2 bar (pressure) and 720 K (temperature). The sulphuric acid thus obtained is 96-98% pure.
7.22 How is SO2 an air pollutant?
Ans- Sulfur dioxide harms the ecosystem in a variety of ways.
- It produces sulfuric acid when it mixes with atmospheric water vapour. Acid rain results from this. Buildings composed of marble are particularly vulnerable to the effects of acid rain.
- Even at low quantities, SO2 induces irritation in the respiratory tract. Additionally, it can irritate the eyes, throat, and larynx, which results in shortness of breath.
- Plants are severely harmed by it. Long-term sulphur dioxide exposure causes plants to lose colour from their leaves. Chlorosis is the name given to this disorder. This occurs as a result of sulphur dioxide’s influence on chlorophyll synthesis.
7.23 Why are halogens strong oxidising agents?
Ans- Halogens typically have an electrical configuration of np5, where n is between 2 and 6. In order to complete their octet and achieve the stable noble gas state, halogens just require one more electron. Additionally, halogens have strong negative electron gain enthalpies and low dissociation energies, making them highly electronegative substances. They therefore have a strong propensity to gain an electron. They therefore function as potent oxidising agents.
7.24 Explain why fluorine forms only one oxoacid, HOF.
Ans- Cl, Br, and I combine to generate the HOX, HOXO, HOXO2, and H0XO3 series of oxo acids. The oxidation states of the halogens in these oxo-adds are + 1, + 3, + 5, and + 7, respectively. F does not, however, produce oxo-acids with the +3, +5, and +7 oxidation states because of its strong electronegativity, tiny size, and lack of d-orbitals. It only produces one oxo-acid (HOF).
7.25 Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Ans- Despite having nearly identical electronegativity values to nitrogen, chlorine rarely forms hydrogen bonds. This is because nitrogen has a higher electron density per unit volume than chlorine due to its smaller size.
As a result, hydrogen bonds form more easily in nitrogen.
In contrast, chlorine has an atomic size that is bigger than that of nitrogen and a lower electron density per volume. Due to this, hydrogen bonds between chlorine do not form easily.
7.26 Write two uses of ClO2.
Ans-
- ClO2 is an excellent bleaching agent. It is a bleaching agent that is 30 times more powerful than Cl2. It is employed in the paper industry as well as the textile sector as a bleaching agent for paper pulp.
- ClO2 is a potent chlorinating and oxidising agent. It functions as a germicide to purify water. It is employed to clean drinking water.
7.27 Why are halogens coloured?
Ans- The majority of halogens are coloured. Halogens absorb radiation in the visible spectrum, which explains this. Valence electrons are then excited to a higher energy area as a result. Each halogen exhibits a different colour because each halogen has a different excitation energy threshold.
7.28 Write the reactions of F2 and Cl2 with water.
Ans- When chlorine gas reacts with water it gives Hydrochloric acid and Hypochlorous acid
Cl2 + H2O → HCl (Hydrochloric Acid) + HOCl (Hypochlorous acid)
Fluorine gas on reaction with water gives Hydrogen ions, Fluorine ions, Oxygen gas and Hydrofluoric acid as products.
2F2 + 2H2O → 4H+ + 4F− + O2 + 4HF
7.29 How can you prepare Cl2 from HCl and HCl from Cl2? Write reactions only.
Ans- Cl2 can be prepared from HCl by Deacon’s process.
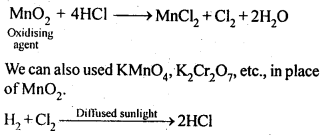
7.30 What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Ans- In an experiment conducted by Neil Bartlett, oxygen and PtF6 reacted to generate O2 +[PtF6], a red-colored molecule.
Neil noticed that the first ionisation energies of xenon and oxygen were nearly identical (about 1170 kJ/mol). He attempted to react Xe and PtF6 as a result, and was successful in producing the crimson complex Xe+[PtF6].
7.31 What are the oxidation states of phosphorus in the following:
(i) H3PO3
(ii) PCl3
(iii) Ca3P2
(iv) Na3PO4
(v) POF3?
Ans- Let the oxidation state of p be x.
(i) H3PO3
3 + x + 3(-2) = 0
3 + x – 6 = 0
x – 3 = 0
x = 3
(ii) PCl3
x + 3(-1) = 0
x – 3 = 0
x = 3
(iii) Ca3P2
3(+2) + 2(x) = 0
6 + 2x = 0
2x = -6
x = -6 / 2
x = -3
(iv) Na3PO4
3(+1) + x + 4(-2) = 0
3 + x – 8 = 0
x – 5 = 0
x = 5
(v) POF3
x + (-2) + 3(-1) = 0
x – 2 – 3 = 0
x – 5 = 0
x = 5
7.32 Write balanced equations for the following:
(i) NaCl is heated with sulphuric acid in the presence of MnO2.
(ii) Chlorine gas is passed into a solution of NaI in water.
Ans- (i) 4NaCl + MnO2 + 4H2SO4 → MnCl2 + 4NaHSO4 + 2H2O + Cl2
Manganese(IV) oxide reacts with sodium chloride and sulfuric acid to produce manganese(II) chloride, chlorine, sodium bisulfate and water. This reaction takes place at a temperature near 100°C.
(ii) Cl2 + NaI → 2NaCl + I2
Chlorine reacts with sodium iodide to produce sodium chloride and iodine. Chlorine – diluted solution. Sodium iodide – cold solution
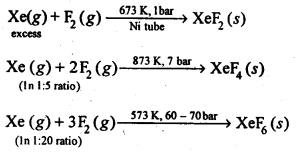
7.33 How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Ans- XeF2, XeF4 and XeF6 are obtained by a direct reaction between Xe and F2. The condition under which the reaction is carried out determines the product.
7.34 With what neutral molecule is ClO– isoelectronic? Is that molecule a Lewis base?
Ans- ClO– is isoelectronic to ClF. Also, both species contain 26 electrons in all as shown.
Total electrons ClO– = 17 + 8 + 1 = 26
In ClF = 17 + 9 = 26
ClF acts like a Lewis base as it accepts electrons from F to form ClF3.
7.35 How are XeO3 and XeOF4 prepared?
Ans-
- XeF4 and XeF6 can be hydrolyzed to produce XeO3 under regulated pH conditions in the reaction medium. The response is:
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2 - 𝑋eO𝐹4 can be prepared on partial hydrolysis of 𝑋eF6 as shown below:
XeF6 + H2O → XeOF4 + 2HF
7.36 Arrange the following in the order of property indicated for each set:
(i) F2, Cl2, Br2, I2 – increasing bond dissociation enthalpy.
(ii) HF, HCl, HBr, HI – increasing acid strength.
(iii) NH3, PH3, AsH3, SbH3, BiH3 – increasing base strength.
Ans-
- As the bond distance grows from F2 to I2, due to an increase in atom size, the bond dissociation enthalpy falls.
The enthalpy required to break an F-F bond is lower than that required to break a Cl-Cl or even a Br-Br bond. This is due to the tiny size of the F atom and the strong electron-electron attraction between lone pairs of electrons in the F2 molecule, which are significantly closer to one another than in the case of Cl2. Bond dissociation enthalpy increases in the following order: I, F2, Br2, and Cl2. - Acid strength of HF, HCI, HBr and HI depends upon their bond dissociation enthalpies. Since the bond dissociation enthalpy of H – X bond decreases from H – F to H-l as the size of atom increases from F to I.
Thus, the acid strength order is HF < HCI < HBr < HI - Because the central atom of NH3, PH3, ASH3, SbH3, and BiH3 has a lone pair of electrons, these molecules behave as Lewis bases. The size of the atom grows from N to Bi. As the core atom’s electron density diminishes, the basic strength from NH3 to BiH3 also declines. BiH3, SbH3, AsH3, PH3, and NH3 are the order of fundamental strength.
7.37 Which one of the following does not exist?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) XeF6
Ans- NeF2 does not exist.
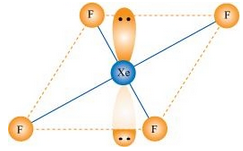
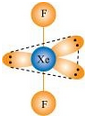
7.38 Give the formula and describe the structure of a noble gas species which is isostructural with:
(i) ICl4 –
(ii) IBr2 –
(iii) BrO3 –
Ans- (i) ICI4– : In ICI4–, central atom I has seven valence electrons and one due to negative charge.
(ii) IBr2– : In IBr2–, central atom I has eight electrons. Two of these are utilized in forming two single bonds with two Br atom. Six remaining electrons constitutes three lone pairs
(iii) In Br03– : ion the central Br atom has 8 valence electrons (7 +1). Out of these, it shares 4 with two atoms of O forming Br = O bonds. Out of the remaining four .electrons, 2 are donated to the third O atom which accounts for its negative charge.
7.39 Why do noble gases have comparatively large atomic sizes?
Ans- Noble gases have relatively high atomic sizes because their electron shells are totally filled, and this fully filled electronic arrangement gives them a stable configuration. The noble gases are larger because electrons are often farther apart, which lessens the electrical repulsion.
Van der Waal’s radius, which is significantly larger than covalent or ionic radii, is what determines the atomic radius of noble gases.
7.40 List the uses of neon and argon gases.
Ans – Neon’s uses
For the purpose of displaying advertisements, neon is utilised in fluorescent and discharge tubes. By combining neon with other gases, “neon signs,” or glows of various colours, can be created. In botanical gardens and green houses, neon bulbs are employed.
Using argon gas
- Gas-filled electric lamps use both argon and nitrogen. Because Ar is more inert than N, this occurs.
- In a high metallurgical process, it is typically employed to create an inert temperature.
It is also used in laboratories to handle materials that are sensitive to air.



