NCERT Exercise Solutions – Chemistry Chapter 8 The d and f Block Elements
8.1 Write down the electronic configuration of:
(i) Cr3+ (iii) Cu+ (v) Co2+ (vii) Mn2+
(ii) Pm3+ (iv) Ce4+ (vi) Lu2+ (viii) Th4+
Ans- (i) Cr3+: 1s2 2s2 2p6 3s2 3p6 3d3 Or, [Ar]183d3
(ii) Pm3+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d105s2 5p6 4f4 Or, [Xe]54 3d3
(iii) Cu+: 1s2 2s2 2p6 3s2 3p6 3d10 Or, [Ar]18 3d10
(iv) Ce4+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 Or, [Xe]54
(v) Co2+: 1s2 2s2 2p6 3s2 3p6 3d7 Or, [Ar]183d7
(vi) Lu2+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d1 Or, [Xe]542f143d3
(vii) Mn2+: 1s2 2s2 2p6 3s2 3p6 3d5 Or, [Ar]18 3d5
8.2 Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
Ans- Electronic configuration of Mn2+ is 3d5. This is a half-filled configuration and hence stable. It is well known that orbitals that are partially and completely filled are more stable. As a result, Mn has a stable d5 structure in the (+2) state. This explains why Mn2+ does not easily oxidise to Mn3+. Additionally, Fe2+ has a 3d6 structure and can transition to a 3d5 shape, which is more stable, by losing one electron. Fe2+ therefore readily undergoes oxidation to Fe+3 oxidation state.
8.3 Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
Ans- In all the elements listed, with the removal of valence 45 electrons (+2 oxidation state), the 3d-orbitals get gradually occupied. Since the number of empty d-orbitals decreases or the number of unpaired electrons in 3d orbitals increases, the stability of the cations (M2+) increases from Sc2+to Mn2+.
| Sc | Ti | V | Cr | Mn | |
| +2 | +2 | +2 | +2 | ||
| +3 | +3 | +3 | +3 | +3 | |
| Oxidation state | +4 | +4 | +4 | +4 | |
| +5 | +5 | +6 | |||
| +6 | +7 |
8.4 To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
Ans- The first half of the transition series’ elements exhibit a wide range of oxidation states, with Mn having the most (+2 to +7). As the atomic number rises, the +2 oxidation state becomes more stable. This occurs as the d-orbital fills up with extra electrons. Sc does not, however, exhibit a +2 oxidation state. It has a 4s23d1 electrical configuration. To become Sc3+, it loses all three of its electrons. Sc’s +3 oxidation state is extremely stable because it achieves a stable noble gas structure, [Ar], by losing all three electrons. For the same reason, Ti (+ 4) and V (+ 5) are quite steady. Because its d-orbital is exactly half-filled, [Ar] 3d5, after losing two electrons, Mn’s +2 oxidation state is exceptionally stable.
8.5 What may be the stable oxidation state of the transition element with the following d electron configurations in the ground state of their atoms : 3d 3 , 3d 5 , 3d 8 and 3d 4?
Ans- Electronic configuration in ground stateStable oxidation states
(i) 3d3 (Vanadium) +2, +3, +4 and +5
(ii) 3d5 (Chromium) +3, +4, +6
(iii) 3d5 (Manganese) +2, +4, +6, +7
(iv) 3d8 (Cobalt) +2, +3
(v) 3d4 There is no3d4 configuration in ground state.
8.6 Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
Ans- Cr2072- and Cr042- (Group number = Oxidation state of Cr = 6).
Mn04– (Group number = Oxidation state of Mn = 7).
8.7 What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Ans- Contraction of the lanthanoids: The 4f-subshell of the lanthanoids is being filled with electrons. The nuclear charge increases when travelling from left to right, and this increase is anticipated to be offset by an increase in the strength of the shielding effect provided by the four f- electrons. The f-electrons, however, have a terrible ability to protect. The result is a decrease in the atomic and ionic radii from left to right, which is known as lanthanoid contraction.
Consequences of Lanthanoid Contraction
(a) Separation of Lanthanoids: Only lanthanoid contraction makes it possible to separate lanthanoids. Since the features of all lanthanoids are fairly similar, it is challenging to distinguish between them. Their characteristics, such as ionic size and capacity to form complexes, however, differ slightly due to lanthanoid contraction.
(b) Variation in basic strength of hydroxides: From La(OH)3 to Lu(OH), the basic strength of oxides and hydroxides falls. The covalent nature of the M—OH bond increases as a result of the shrinkage of M3+ ions caused by lanthanoid contraction. The O—H bond cleavage-related acidic strength exhibits the opposite tendency, i.e., an increase along the series.
(c) Similarly in the atomic sizes of the elements of the second and third transition series present in the same group: We know that the atomic sizes of the elements generally increase appreciably down a group. Similar trend is also expected in the elements present in the different groups of d-block.
(d) Variation in standard reduction potential: Due to lanthanoid contraction there is a small but steady increase in the standard reduction potential (E°) for the reduction process.
M3+ (aq) + 3e– → M (aq)
(e) Variation in physical properties like melting point, boiling point, hardness etc: Numerous physical characteristics, including m.pt., b.pt., hardness, etc., rise as the atomic number rises. This is due to the fact that when size decreases, the forces of attraction between the atoms grow stronger.
8.8 What are the characteristics of the transition elements and why are they called transition elements? Which of the d-block elements may not be regarded as the transition elements?
Ans- General characteristics of transition elements.
(i)Electronic configuration – (n -1) d1-10 ns1-2
(ii)Metallic character – With the exceptions of Zn, Cd and Hg, they have typical metallic structures.
(iii)Atomic and ionic size-ions of same charge in a given series show progressive decrease in radius with increasing atomic number.
(iv)Oxidation state-Variable; ranging from+2 to +7.
(v)Paramagnetism – The ions with unpaired electrons are paramagnetic.
(vi)Ionisation enthalpy – Increases with increase in charge.
Formation of coloured ions – Due to presence of unpaired electrons.
(viii) Formation of complex compounds – Due to small size and high charge density of metal ions.
(ix)They possess catalj^c properties – Due to
their ability to adopt multiple oxidation states. .
(x)Formation of interstitial compounds.
8.9 In what way is the electronic configuration of the transition elements different from that of the non transition elements?
Ans- The d-orbital of transition metals is only partially filled. Consequently, (n – 1)d1-10 ns0-2 is the electrical configuration of transition components.
The non-transition elements either have no d-orbital or have a d-orbital that is completely filled. As a result, non-transition elements have an electrical configuration of ns1-2 or ns2np1-6.
8.10 What are the different oxidation states exhibited by the lanthanoids?
Ans- +3 is the common oxidation state of the lanthanoids.
In addition to +3, oxidation states +2 and +4 are also exhibited by some of the lanthanoids.
8.11 Explain giving reasons:
(i) Transition metals and many of their compounds show paramagnetic behaviour.
(ii) The enthalpies of atomisation of the transition metals are high.
(iii) The transition metals generally form coloured compounds.
(iv) Transition metals and their many compounds act as good catalyst.
Ans- (i)The existence of unpaired electrons, each of which possesses a magnetic moment due to its spin angular momentum, causes the emergence of magnetic fields. Transition metals exhibit paramagnetic behaviour because they contain many unpaired d-electrons in their ground state or ionised state.
(ii) Due to the involvement of a bigger number of electrons from (n – l)d in addition to the ns electrons, transition metals have extremely high interatomic metallic interaction. Because more half-filled orbitals are overlapping, the bonding that results from having more valence electrons is stronger. Therefore, more energy is needed to dissolve these metallic connections. As a result, the transition metal’s enthalpy of atomization is quite high.
(iii) The excitation of an electron from a lower energy d-orbital to a higher energy d orbital is what gives transition metal complexes their colour. The perceived colour matches the complementary colour of the absorbed light, and the energy of excitation matches the frequency of the light (whose frequency lies on the visible region). Due to the vacant d-orbitals for the e’ d-d transition, which produces the colour, transition metals can create coloured compounds.
(iv) Transition metals’ capacity to take on many oxidation states and form complexes is thought to be the cause of their catalytic activity. Bonds are formed between the atoms of the catalyst’s surface and the molecules of the reactants when the catalyst is on a solid surface.
8.12 What are interstitial compounds? Why are such compounds well known for transition metals?
Ans- Many interstitial compounds are created by transition metals. They can form weak connections with tiny atoms of elements like H, G, N, and B by trapping them in their crystal lattice.
Their malleability and ductility are reduced when interstitial compounds accumulate, while their tensile strength improves. Due to trapped carbon atoms in interstitial regions, steel and cast iron are harder than wrought iron.
8.13 How is the variability in oxidation states of transition metals different from that of the non transition metals? Illustrate with examples.
Ans- By eliminating all of the valence electrons from transition elements, the oxidation state can change from +1 to the highest oxidation state. Additionally, the oxidation states of transitional elements (Cu+ and Cu2+; Fe2+ and Fe3+) differ by one. The oxidation states of non-transition elements differ by two, for instance, +2 and +4 or +3 and +5, etc.
8.14 Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Ans- Preparation from chromite: Potassium dichromate is generally prepared from chromite ore (FeCr2O4). It is in fact, a mixed oxide Fe0.Cr2O3 of iron and chrome also called ferrochrome or chrome iron.
Preparation of sodium chromate
4FeCr2O4 + 16NaOH + 7O2 → 8NaCrO4 + 2Fe2O3 + 8H2O
Conversion of sodium chromate into sodium dichromate
2Na2CrO4 + conc.H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
Conversion of sodium dichromate to potassium dichromate
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
The solution of potassium dichromate (K2Cr2O7) in water is orange in colour. On increasing the pH i.e. on adding the base, the potassium dichromate changes to potassium chromate (K2CrO4) which is yellow in colour. Thus, on increasing the pH, the colour of the solution changes from orange to yellow.
8.15 Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(i) iodide
(ii) iron(II) solution and
(iii) H2S
Ans- K2Cr2O7 acts as a very strong oxidising agent in the acidic medium.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr(SO4)3 + 4H2O + 3[O]
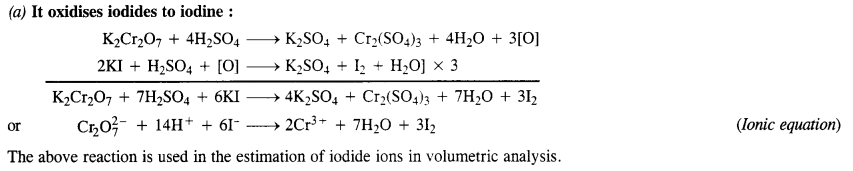


8.16 Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with
(i) iron(II) ions (ii) SO2 and (iii) oxalic acid?
Write the ionic equations for the reactions.
Ans- The mineral pyrolusite can be used to make potassium permanganate (MnO2). K2MnO4 is created when the ore and KOH combine in the presence of either oxygen from the air or an oxidising agent like KNO3 or KClO4.

8.17 For M2+/M and M3+/M2+ systems the E V values for some metals are as follows:
Cr2+/Cr -0.9V Cr3 /Cr2+ -0.4 V
Mn2+/Mn -1.2V Mn3+/Mn2+ +1.5 V
Fe2+/Fe -0.4V Fe3+/Fe2+ +0.8 V
Use this data to comment upon:
(i)the stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+ and
(ii) the ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Ans- (i) The reduction potential of Cr3+/Cr2+ is negative. As a result, Cr3+ cannot be converted to Cr2+. A significant positive reduction potential exists for Mn3+/Mn2+. Consequently, it is simple to convert Mn3+ to Mn2+. The positive reduction potential of Fe3+/Fe2+ is modest. Fe3+ is therefore less stable than Cr3+ but more stable than Mn3+.
(ii) The supplied pairings’ reduction potentials rise in the following order. Mn2+/Mn, Cr2+/Cr, Fe2+/Fe
Therefore, oxidising Fe to Fe2+ is more difficult than oxidising Cr to Cr2+ or Mn to Mn2+. The value for Fe3+/Fe2+ is higher than that for Cr3+/Cr2+ and lower than that for Mn3+/Mn2+, allowing these metals to be grouped in the following order: Fe Cr Mn. Therefore, reducing Fe3+ to Fe2+ is simpler than reducing Mn3+ to Mn2+, but not as simple as reducing Cr3+ to Cr2+. Fe3+ is therefore less stable than Cr3+ but more stable than Mn3+.
8.18 Predict which of the following will be coloured in aqueous solution?
Ti 3+, V3+ , Cu+ , Sc3+, Mn2+, Fe3+ and Co2+.
Give reasons for each.
Ans- The capacity of e’ to hop from a lower energy d orbital to a higher energy d orbital causes the ions Ti3+, V3+ Mn2+, Fe3+, and CO2+ to exhibit colour in their aqueous solutions. This d-d transition is not possible for the ions Cu+ and Sc3+ either because there is no e- in the 3d orbital or because the d orbital is completely filled.
8.19 Compare the stability of +2 oxidation state for the elements of the first transition series.
Ans- The common oxidation state for elements in the 3d series is + 2, which results from the involvement of only 4s electrons. Due to the pairing of electrons in the third subshell, the propensity to exhibit the greatest oxidation state increases from Sc to Mn and subsequently decreases. Ti(II) is less stable than Ti, hence Sc(II) does not exist in the series (IV). Zn only has a +2 oxidation state at the other end of the series.
8.20 Compare the chemistry of actinoids with that of the lanthanoids with special reference to:
(i) electronic configuration (iii) oxidation state
(ii) atomic and ionic sizes and (iv) chemical reactivity.
Ans- (i) Electronic configuration: Actinoids have an electronic configuration of [Rn]86 5f0-14 6d0-1 7s2 compared to lanthanoids’ [Xe]54 4f1-14 5d0-1 6s2. Lanthanoids are part of the 4 f series, whereas actinoids are part of the 5 f series.
(ii) Atomic and ionic sizes: As we move from left to right, both lanthanoids and actinoids exhibit a decrease in the size of their atoms or ions in the + 3 oxidation state. Actinoids refer to the decline as actinoid contraction, whereas lanthanoids refer to it as lanthanoid contraction. Due to less effective 5F electron shielding, the contractility varies more from element to element in actinodes.
(iii) Atomic and lonic dimensions: Actinoids also display actinoid contraction, much like lanthanoids do (overall decrease in atomic and ionic radii). Because 5f orbitals have a poor shielding effect, the shrinkage is more pronounced.
(iv) Reactivity of chemicals: The lanthanide series’ earliest members are more reactive than later ones. They behave similarly to calcium in terms of reactivity. The lanthanides begin to behave like aluminums as their atomic number rises. On the other hand, actinoids are extremely reactive metals, particularly when they are finely split. They produce a combination of oxide and hydride when added to boiling water.
8.21 How would you account for the following:
(i) Of the d 4 species, Cr2+ is strongly reducing while manganese(III) is strongly oxidising.
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.
(iii) The d 1 configuration is very unstable in ions.
Ans- (a) The E° value of Mn3+/Mn2+ is positive (+ 157 V), but that of Cr3+/Cr2+ is negative (-041 V). This indicates that Mn3+ ions can take electrons and operate as oxidising agents, but Cr2+ ions can lose electrons to generate Cr3+ ions.
(b) Cobalt (II) is stable in aqueous solution, but it changes its oxidation state from +2 to +3 in the presence of a complexing agent and is quickly oxidised.
(c) The ion in the d1 configuration is predicted to be incredibly unstable and has a strong desire to change its configuration to the more stable d° form by letting go of the lone electron in the d-subshell.
8.22 What is meant by ‘disproportionation’? Give two examples of disproportionation reaction in aqueous solution.
Ans- Disproportionation reactions occur when the same substance goes through both oxidation and reduction, or when the oxidation number of an element changes to produce two distinct products.

8.23 Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
Ans- Cu has a +1 oxidation state and produces the Cu+ ion when its configuration is [Ar] 4s13d10 because losing one electron allows the cation to gain a stable configuration of d-orbitals (3d10).
8.24 Calculate the number of unpaired electrons in the following gaseous ions:
Mn3+ , Cr3+, V3+ and Ti 3+.
Which one of these is the most stable in aqueous solution?
Ans-
| Gaseous ions | Number of unpaired electrons | |
| (i) | Mn3+ , [Ar] 3d4 | 4 |
| (ii) | Cr3+ , [Ar] 3d3 | 3 |
| (iii) | V3+ , [Ar] 3d2 | 2 |
| (iv) | Ti3+ , [Ar] 3d1 | 1 |
8.25 Give examples and suggest reasons for the following features of the transition metal chemistry:
(i) The lowest oxide of transition metal is basic, the highest is amphoteric/acidic.
(ii) A transition metal exhibits highest oxidation state in oxides and fluorides.
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
Ans- (i) Lower transition metal oxides are basic because they include metal atoms in a low oxidation state, whereas higher ones are acidic because they contain metal atoms in a high oxidation state. As an illustration, MnO is basic while Mn2O7 is acidic. Lower oxidation state oxides are ionic and therefore basic. Higher oxidation state oxides are covalent and therefore acidic.
(ii) Because oxygen and fluorine are extremely electronegative, tiny, and powerful oxidising agents, transition metals display greater oxidation states in oxides and fluorides. Vanadium, for instance, exhibits an oxidation state of +5 in V2O5, and osmium exhibits an oxidation state of +6 in O5F6.
(iii) Oxygen is a strong oxidising agent due to its high electronegativity and small size. So, oxo-anions of a metal have the highest oxidation state. For example, in MnO-4, the oxidation state of Mn is +7.
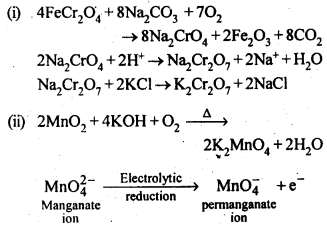
8.26 Indicate the steps in the preparation of:
(i) K2Cr2O7 from chromite ore.
(ii) KMnO4 from pyrolusite ore.
Ans–
8.27 What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
Ans- A homogenous blend of two or more metals, or metals and non-metals, is referred to as an alloy. A significant lanthanoid metal alloy is mischmetal, which has a composition of 50% cerium, 25% lanthanum, and trace amounts of Nd (neodymium), and Pr (Praseody-mium). It is used to make lighter flints, bullets, and shells from Mg-based alloy.
8.28 What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements : 29, 59, 74, 95, 102, 104.
Ans- Inner-transition elements are those in the f-block where the final electron enters the f-sub shell. These include actinoids (Z=90–103) and lanthanoids (Z=58–71). As a result, the inner transition elements are the elements with atomic numbers 59, 95, and 102.
8.29 The chemistry of the actinoid elements is not so smooth as that of the lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
Ans- The three most common oxidation states for lanthanoids are (+2, +3, +4). The most prevalent of these oxidation states is the +3 state. The few oxidation states that lanthanoids exhibit are due to the enormous energy gap between the 4f, 5d, and 6s orbitals. However, there isn’t much of a difference in energy between the 5f, 6d, and 7s orbitals. Actinoids exhibit a wide range of oxidation states as a result. For instance, although neptunium exhibits +3, +4, +5, and +7 oxidation states, uranium and plutonium exhibit +3, +4, +5, and +6. In the case of actinoids, +3 is also the most prevalent oxidation state.
8.30 Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
Ans- Lawrencium (Lr = 103); [Rn] 5f146d17s2 oxidation state = +3.
8.31 Use Hund’s rule to derive the electronic configuration of Ce3+ ion, and calculate its magnetic moment on the basis of ‘spin-only’ formula.
Ans-

8.32 Name the members of the lanthanoid series which exhibit +4 oxidation states and those which exhibit +2 oxidation states. Try to correlate this type of behaviour with the electronic configurations of these elements.
Ans- The lanthanides that exhibit +2 and +4 states are shown in the given table. The atomic numbers of the elements are given in the parenthesis.
| +2 | +4 |
| Nd(60) | Ce(58) |
| Sm(62) | Pr(59) |
| Eu(63) | Nd(60) |
| Tm(69) | Tb(65) |
| Yb(70) | Dy(66) |
- Ce after forming Ce4+ attains a stable electronic configuration of [Xe].
- Tb after forming Tb4+ attains a stable electronic configuration of [Xe] 4f7.
- Eu after forming Eu2+ attains a stable electronic configuration of [Xe] 4f7.
- Yb after forming Yb2+ attains a stable electronic configuration of [Xe] 4f14.
8.33 Compare the chemistry of the actinoids with that of lanthanoids with reference to:
(i) electronic configuration
(ii) oxidation states and
(iii) chemical reactivity.
Ans- (i)Electronic configuration: Actinoids have an electronic configuration of [Rn]86 5f0-14 6d0-1 7s2 compared to lanthanoids’ [Xe]54 4f1-14 5d0-1 6s2. Lanthanoids are part of the 4 f series, whereas actinoids are part of the 5 f series.
(ii) Atomic and ionic sizes: As we move from left to right, both lanthanoids and actinoids exhibit a decrease in the size of their atoms or ions in the + 3 oxidation state. Actinoids refer to the decline as actinoid contraction, whereas lanthanoids refer to it as lanthanoid contraction. Due to less effective 5F electron shielding, the contractility varies more from element to element in actinodes.
(iii) Reactivity of chemicals: The lanthanide series’ earliest members are more reactive than later ones. They behave similarly to calcium in terms of reactivity. The lanthanides begin to behave like aluminums as their atomic number rises. On the other hand, actinoids are extremely reactive metals, particularly when they are finely split. They produce a combination of oxide and hydride when added to boiling water.
8.34 Write the electronic configurations of the elements with the atomic numbers 61, 91, 101, and 109.
Ans- Z=61 (Promethium, Pm) [Xe]544f55d0 6s2
Z = 91 (Protactinium, Pa) => [Rn]86 5f2 6d1 7s2
Z = 101 (Mendelevium, Md)=> [Rn]86 5f13 6d0 7s2
Z = 109 (Meitnerium, Mt) [Rn]86 5f14 6d7 7s2
8.35 Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
(i) electronic configurations (ii) oxidation states
(iii) ionisation enthalpies and (iv) atomic sizes.
Ans – (i) In the 1st, 2nd and 3rd transition series, the 3d, 4d and 5dorbitals are respectively filled. We know that elements in the same vertical column generally have similar electronic configurations.
In the first transition series, two elements show unusual electronic configurations:
Cr(24) = 3d5 4s1
Cu(29) = 3d10 4s1
Similarly, there are exceptions in the second transition series. These are:
Mo(42) = 4d5 5s1
Tc(43) = 4d6 5s1
Ru(44) = 4d7 5s1
Rh(45) = 4d8 5s1
Pd(46) = 4d10 5s0
Ag(47) = 4d10 5s1
There are some exceptions in the third transition series as well. These are:
W(74) = 5d4 6s2
Pt(78) = 5d9 6s1
Au(79) = 5d10 6s1
As a result of these exceptions, it happens many times that the electronic configurations of the elements present in the same group are dissimilar.
(ii) In each of the three transition series the number of oxidation states shown by the elements is the maximum in the middle and the minimum at the extreme ends.
However, +2 and +3 oxidation states are quite stable for all elements present in the first transition series. All metals present in the first transition series form stable compounds in the +2 and +3 oxidation states. The stability of the +2 and +3 oxidation states decreases in the second and the third transition series, wherein higher oxidation states are more important.
For example are stable complexes, but no such complexes are known for the second and third transition series such as Mo, W, Rh, In. They form complexes in which their oxidation states are high. For example: WCl6, ReF7, RuO4, etc.
(iii) The first ionisation enthalpy rises from left to right in each of the three transition series. There are a few exceptions, though. In comparison to the first and second transition series, the third transition series has higher initial ionisation enthalpies. This happens as a result of 4felectrons’ subpar shielding effect in the third transition series.
In comparison to elements belonging to the same vertical column in the first transition series, some elements in the second transition series have higher first ionisation enthalpies. Additionally, some elements in the second transition series have first ionisation enthalpies that are lower than those of the elements in the first transition series that belong to the same vertical column.
(iv) Over a time, atomic size typically decreases from left to right. The atomic sizes of the elements belonging to the same vertical column in the second transition series of the three transition series are now larger than those of the elements belonging to the same vertical column in the first transition series. The elements in the third transition series, however, have nearly identical atomic sizes to their corresponding counterparts in the second transition series. The contraction of the lanthanoid is to blame.
8.36 Write down the number of 3d electrons in each of the following ions:
Ti 2+, V2+ , Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+ and Cu2+.
Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral).
Ans– For the explanation of the involvement of 3d orbitals in the hydrated ions (octahedral in nature) consult the next unit on coordination compounds.
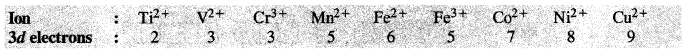
8.37 Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
Ans- The fourth (Ad), fifth (5d), and sixth (6d) transition series contain the larger transition components. Due to the following factors, their properties are anticipated to differ from those of the components in the first (3d) series:
(i) Because there are more electron shells in the elements from the Ad and 5d series, their atomic radii are larger. However, due to lanthanoid contraction, there is far less of a difference between Ad and 5d transition elements.
(ii) The m.p. and b.p. of the elements in the Ad and 5d series are higher as a result of stronger interatomic bonding.
(iii)As we go from one series to the next, it is predicted that ionisation enthalpies will drop. The values for the components of the 5d series, however, are higher.
8.38 What can be inferred from the magnetic moment values of the following complex species ? Example Magnetic Moment (BM) K4[Mn(CN)6) 2.2 [Fe(H2O)6] 2+ 5.3 K2[MnCl4] 5.9
Ans-
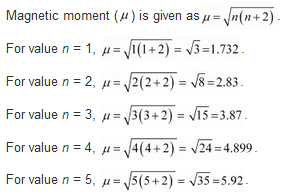
(i) K4[Mn(CN)6]
Oxidation state of Mn : [Mn(CN)6]4- , x + 6(-l) = -4 or x = -4 + 6 = + 2
The magnetic value of 1·73 B.M. indicates the presence of one unpaired electron in the complex. When six, CN– ions (or ligands) approach Mn2+ ion, electrons in 3d orbitals

(ii) [Fe(H2O)6]2+

We can see from the above calculation that the given value is closest to n = 4. Also, in this complex, Fe is in the +2 oxidation state. This means that Fe has 6 electrons in the d-orbital.
Hence, we can say that H2O is a weak field ligand and does not cause the pairing of electrons.
(iii) K2[MnCl4]

We can see from the above calculation that the given value is closest to n = 5. Also, in this complex, Mn is in the +2 oxidation state. This means that Mn has 5 electrons in the d-orbital.


