NCERT Exercise Solutions – Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
6.1 Copper can be extracted by hydrometallurgy but not zinc. Explain.
Ans– The reduction potentials of zinc and iron are lower than that of copper. In hydrometallurgy, zinc and iron can be used to displace copper from their solution.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
But to displace zinc, more reactive metals i.e., metals having lower reduction potentials than zinc such as Mg, Ca, K, etc. are required. But all these metals react with water with the evolution of H2 gas.
2K(s) + 2H2O(l) → 2KOH(aq) + H2(g)
But with water, these metals (Al, Mg, Ca and K) forms their corresponding ions with the evolution of H2 gas.
Thus, Al, Mg, Ca, K, etc., cannot be used to displace zinc from zinc solution, and only copper can be extracted by hydrometallurgy but not the zinc.
6.2 What is the role of depressant in froth floatation process?
Ans- The depressants’ function in the froth floatation process is to separate two sulphide ores by specifically preventing one ore from generating froth. For instance, NaCN is employed as a depressant to selectively allow PbS to come with froth but prohibit ZnS from coming to froth in order to separate two sulphide ores (ZnS and Pbs). NaCN interacts with ZnS to generate Na2[Zn(CN)4], which is what causes this to occur.
4 NaCN + ZnS → Na2[Zn(CN)4] + Na2S
6.3 Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Ans-
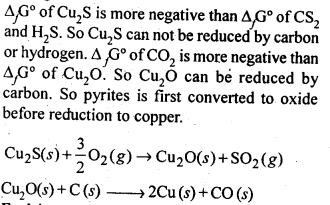
6.4 Explain:
(i) Zone refining
(ii) Column chromatography.
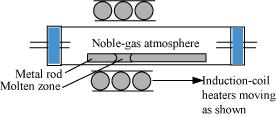
Ans- (i) Zone refining: This technique is utilised to produce semiconductors as well as other very pure metals including Ge, Si, B, Ca, and In. The idea behind it is that impurities are more soluble in a metal’s molten state (melt) than in its solid state. A rotating circular heater is used to heat the impure metal in the form of a bar at one end. The heater is gradually moved the entire length of the rod. While the impurities travel into the nearby molten zone, the pure metal separates out of the melt. Until all of the impurities are concentrated at one end of the rod, this process is repeated numerous times. At that point, the end of the rod is cut off and discarded.
(ii) Column chromatography: The technique of column chromatography is used to separate the various components of a mixture. It is a highly helpful method for purifying elements that are only found in trace amounts. Additionally, contaminants that share many chemical characteristics with the element to be purified are eliminated using this method. The foundation of chromatography is the idea that various elements of a mixture are adsorbed on an adsorbent in various ways. Mobile phase and stationary phase are the two phases of chromatography. Immobile and immiscible, the stationary phase. Typically, the stationary phase in column chromatography uses an Al2O3 column. The sample extract may be dissolved in the mobile phase, which might be a gas, liquid, or supercritical fluid.
6.5 Out of C and CO, which is a better reducing agent at 673 K?
Ans- At 673 K, the value of ΔG(co,co2) is less than that of ΔG (c,co). Therefore, CO can be oxidised more easily to CO2 than C to CO. Hence, CO is a better reducing agent than C at 673 K.
6.6 Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
Ans- Metals like Ag, Au, Pt, and others found in anode mud are less reactive than Cu. Even though they make up the electrode that serves as the anode, they are actually not in a position to lose electrons. While the full amount of copper present takes part in the oxidation half reaction, all of these metals are left as residue under the anode (known as anode mud).
Cu(s) → Cu2+(aq) + 2e–
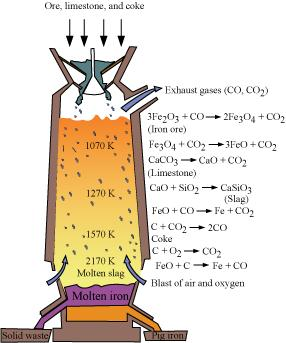
6.7 Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Ans- During the extraction of iron, the reduction of iron oxides takes place in the blast furnace. In this process, hot air is blown from the bottom of the furnace and coke is burnt to raise the temperature up to 2200 K in the lower portion itself. The temperature is lower in the upper part. Thus, it is the lower part where the reduction of iron oxides (Fe2O3 and Fe3O4) takes place.

6.8 Write chemical reactions taking place in the extraction of zinc from zinc blende.
Ans- When zinc is being extracted from zinc blende, the following procedures are When zinc is being extracted from zinc blende, the following procedures are used:
(i) Concentration: After crushing the zinc blende ore, the concentration is carried out using a froth-floatation technique.
(ii) Roasting: The concentrated ore is then heated to roughly 1200 K while being exposed to extra air, causing zinc oxide to develop. 2ZnS + 3O2 → 2ZnO + 2SO2
(iii) Extraction of zinc from zinc oxide (Reduction): Zinc is extracted from zinc oxide by the process of reduction. The reduction of zinc oxide is carried out by mixing it with powdered coke and then, heating it at 673 K. ZnO + C → Zn + CO
(iv) Electrolytic Refining Zinc can be refined by the process of electrolytic refining. In this process, impure zinc is made the anode while a pure copper strip is made the cathode. The electrolyte used is an acidified solution of zinc sulphate (ZnSO4). Electrolysis results in the transfer of zinc in pure from the anode to the cathode.
- Anode: Zn → Zn2+ + 2e–
- Cathode : Zn2+ + 2e– → Zn
6.9 State the role of silica in the metallurgy of copper.
Ans- In the metallurgy of copper, silica (SiO2) functions as an acidic flux and reacts with FeO (the primary impurity) to generate FeSiO3, which is a slag.
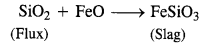
6.10 Which method of refining may be more suitable if element is obtained in minute quantity?
Ans- Chromatography is a method used to separate, purify, identify, and characterise the different parts of a mixture, whether it contains colour or not. Because the technique was initially employed for the separation of coloured substances (plant pigments) into their constituent components, the term “chromatography” originally came from the Greek words “chroma,” which means colour, and “graphy,” which means writing.
6.11 Which method of refining will you suggest for an element in which impurities present have chemical properties close to the properties of that elements?
Ans- The adsorbent serves as the stationary phase in chromatography, especially adsorption chromatography. For better outcomes, it needs to meet specific requirements.
(i) It should have a strong but focused adsorption capacity.
(ii) The particles must to be uniformly sized and spherical in shape.
(iii) The adsorbent must not undergo chemical reactions with the elution solvents or the mixture’s constituent parts.
(iv) As little soluble material as feasible should be present in the adsorbent.
(v) The adsorbent needs to have a neutral surface and be catalytically inactive.
(vi) The adsorbent needs to be accessible.
(vi) The adsorbent must be crystal-clear white.
6.12 Describe a method for refining nickel.
Ans- Impurities are left behind when impure nickel is heated to 330–350 K in the presence of CO and generates volatile nickel tetracarbonyl. The resulting nickel tetracarbonyl is then heated to a higher temperature (450–470 K), where it goes through thermal breakdown to yield pure nickel.

6.13 How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
Ans- To separate alumina from silica in bauxite ore associated with silica, first the powdered ore is digested with a concentrated NaOH solution at 473 − 523 K and 35 − 36 bar pressure. This results in the leaching out of alumina (Al2O3) as sodium aluminate and silica (SiO2) as sodium silicate leaving the impurities behind.
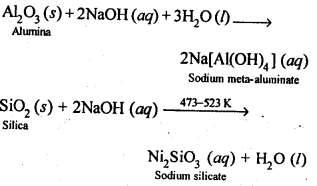
The resulting solution is filtered to remove the undissolved impurities, sodium meta-aluminate can be precipitated as hydrated aluminium oxide by passing CO2 vapours. The sodium silicate formed cannot be precipitated and can be filtered off.
6.14 Giving examples, differentiate between ‘roasting’ and ‘calcination’.
Ans– Roasting – Sulphide ores are heated in a steady stream of air at a temperature below the melting point of the metal during roasting, which turns them into oxides. For instance, using this method, the corresponding oxides of Zn, Pb, and Cu are produced from their sulphide ores.
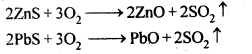
On the other hand, calcination is the process of converting hydroxide and carbonate ores to oxides by heating the ores either in the absence or in a limited supply of air at a temperature below the melting point of the metal. This process causes the escaping of volatile matter leaving behind the metal oxide. For example, hydroxide of Fe, carbonates of Zn, Ca, Mg are converted to their respective oxides by this process.
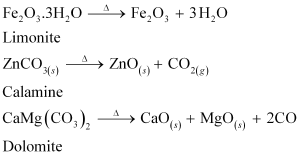
6.15 How is ‘cast iron’ different from ‘pig iron’?
Ans- Pig iron is the name for the iron produced in blast furnaces. It has several impurities like S, P, Si, and Mn in minor proportions together with about 4% carbon.
Pig iron and coke are melted together using a hot air blast to produce cast iron. Compared to pig iron, it has less carbon (3%) than that material. Cast iron is much harder and brittler than pig iron.
6.16 Differentiate between “minerals” and “ores”.
Ans- Minerals are the naturally occurring materials in which metals or their compounds can be found in the earth.
Ores: The minerals that can cheaply and conveniently be used to extract metals are referred to as ores.
6.17 Why copper matte is put in silica lined converter?
Ans- Cu2S and FeS are found in copper matte. To remove the remaining FeO and FeS that are present in the copper matte as slag, the matte is placed in a silica-lined converter (FeSiO3). The silica-lined converter also gets some silica added to it. A hot air burst is then released. As a result, Cu2S is transformed into metallic copper while the leftover FeS and FeO are transformed into iron silicate (FeSiO3).
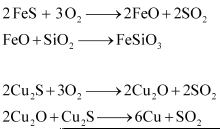
6.18 What is the role of cryolite in the metallurgy of aluminium?
Ans- (a) It reduces the bath’s fusion (melting) point from 2323 K to roughly 1140 K.
(b) It makes alumina a good electrical conductor.
6.19 How is leaching carried out in case of low grade copper ores?
Ans- In case of low grade copper ores, leaching is carried out using acid or bacteria in the presence of air. In this process, copper goes into the solution as Cu2+ ions.
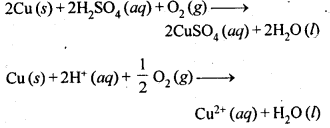
6.20 Why is zinc not extracted from zinc oxide through reduction using CO?
Ans- When ZnO is formed from Zn, the standard Gibbs free energy is smaller than when CO2 is formed from CO. ZnO cannot be converted from ZnO to Zn by CO. Thus, CO reduction cannot be used to recover Zn from ZnO.
The chemical reaction involving the reduction of ZnO by CO is :
ZnO(s) + CO(g) → Zn(s) + CO2(g)
6.21 The value of ∆fG 0 for formation of Cr2 O3 is – 540 kJmol−1and that of Al2 O3 is – 827 kJmol−1 . Is the reduction of Cr2 O3 possible with Al?
Ans- The value of ΔfGø for the formation of Cr2O3 from Cr (-540 kJmol-1) is higher than that of Al2 O3 from Al (-827 kJmol-1). Therefore, Al can reduce Cr2O3 to Cr. Hence, the reduction of Cr2O3 with Al is possible.
Alternatively,
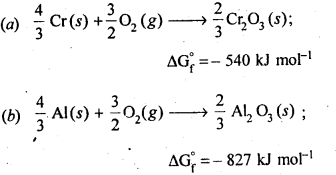
6.22 Out of C and CO, which is a better reducing agent for ZnO?
Ans- The two reduction reactions are:
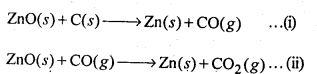
Typically, ZnO is reduced to Zn at a temperature of 1673 K. In the preceding figure, it can be seen that the Gibbs free energy of the creation of CO from carbon is lower than that of ZnO above 1073 K and that of the formation of CO2 from carbon is lower above 1273 K. As a result, C can easily convert ZnO to Zn.
In contrast, the Gibbs free energy required to produce CO2 from CO is always more than that required to form ZnO. CO cannot therefore decrease ZnO. Therefore, C works better as a reducing agent to dissolve ZnO than CO does.
6.23 The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
Ans–
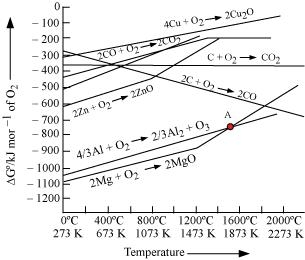
Using an Ellingham diagram, we can research the selection of a reducing agent in a certain situation.
The diagram clearly shows that metals with more negative standard free energies for the formation of their oxides can reduce metals with more positive standard free energies for the creation of their respective oxides. Any metal, according to the Ellingham diagram, will diminish the oxides of other metals that are above it. This is due to the fact that the combined redox reaction’s standard free energy change (rG°) will be negative by an amount equal to the difference between the f G°s of the two metal oxides. Al and Zn may both reduce FeO to Fe in this way, while Fe cannot decrease Al203.
6.24 Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Ans- Chlorine is produced as a by-product of the down process, which is used to make sodium metal. A fused mixture of NaCl and CaCl2 is electrolyzed in this procedure at a temperature of 873 K. At the cathode, sodium is released, and as a byproduct, Cl2 is produced at the anode.
In the electrolysis of molten NaCl, Cl2 is obtained at the anode as a by product.
NaCl(melt) → Na+(melt) + Cl–(melt)
At cathode: Na+(melt) + e– → Na(s)
At anode: Cl–(melt) → Cl(g) + e–
2Cl(g) → Cl2(g)
If an aqueous solution of NaCl is electrolyzed, Cl2 will be obtained at the anode but at the cathode, H2 will be obtained (instead of Na). This is because the standard reduction potential of Na (E°= – 2.71 V) is more negative than that of H2O (E° = – 0.83 V). Hence, H2O will get preference to get reduced at the cathode and as a result, H2 is evolved.
NaCl(aq) → Na+(aq) + Cl–(aq)
6.25 What is the role of graphite rod in the electrometallurgy of aluminium?
Ans- Oxygen gas is evolved at the anode in the electrometallurgy of aluminium. Carbon monoxide and carbon dioxide are produced when oxygen combines with graphite or carbon (graphite electrodes). Al will be wasted if some other metal electrodes are used as the anode because oxygen will react with the aluminium that is created throughout the process to create aluminium oxide (Al2O3), which will enter the reaction mixture. Since graphite is less expensive than aluminium, its wastage is acceptable.
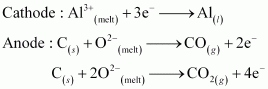
6.26 Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
Ans- (i) Zone refining: This technique is utilised to produce semiconductors as well as other very pure metals including Ge, Si, B, Ca, and In.
Its foundation is the idea that impurities are more soluble in a metal’s molten state (melt) than in its solid state.
A rotating circular heater is used to heat the impure metal in the form of a bar at one end. Pure metal crystallises out of the melt while impurities pass into the nearby molten zone as the heater is steadily pushed along the length of the rod. This procedure is carried out repeatedly until all contaminants are forced to one end of the rod, which is then severed and discarded.
(ii) Electrolytic refining: This method involves using electricity to refine impure metals. In this procedure, a strip of impure metal serves as the cathode and an impure metal strip serves as the anode. The electrolyte is a solution of a soluble salt of the same metal. When an electric current is passed, the electrolyte’s metal ions deposit as pure metal at the cathode while the anode’s impure metal dissolves as ions into the electrolyte. Under the anode, the impurities in the impure metal are gathered. It is referred to as anode mud.
(iii) Vapour-phase refining: This method transforms the crude metal into a suitable volatile compound by heating it with a particular reagent at a lower temperature, and then decomposes the volatile compound at a higher temperature to get the pure metal.
- Mond’s process: Impurities are left behind when impure nickel is heated in a current of CO at 330–350 K because the impurities are transformed into a volatile nickel tetracarbonyl complex. The complex subsequently undergoes thermal breakdown to produce pure nickel after being heated to a higher temperature (450–470K).
- Van Arkel method: This method is Used for preparing ultra-pure metals by removing all the oxygen and nitrogen present as impurities in metals like zirconium and titanium (which are used in space technology).Crude Zr is heated in an evacuated vessel with iodine at 870 K. Zirconium tetraiodide thus formed is separated. It is then decomposed by heating over a tungsten filament at 1800 – 2075 K to give pure Zr.
6.27 Predict conditions under which Al might be expected to reduce MgO.
Ans- The standard Gibbs free energy of forming Al2O3 from Al is lower than that of forming MgO from Mg above 1350°C. Al can so decrease MgO above 1350 degrees Celsius.





