NCERT Exercise Solutions – Chemistry Chapter 9 Coordination Compounds
9.1 Explain the bonding in coordination compounds in terms of Werner’s postulates.
Ans- The following are Werner’s postulates that explain the bonding in coordination compounds:
(i) A metal demonstrates primary and secondary valencies, two different sorts of valencies. Negative ions satisfy primary valencies, while neutral and negative ions can both satisfy secondary valencies. (In current parlance, the primary valency is equivalent to the metal ion’s oxidation number, whereas the secondary valency is equivalent to the metal ion’s coordination number.
(ii) Around the main atom, a metal ion has a certain number of secondary valencies. Additionally, these valencies project in a certain direction in the area designated by the coordination compound’s precise geometry.
(iii) In contrast to secondary valencies, primary valencies are typically ionizable.
9.2 FeSO4 solution mixed with (NH4)2SO4 solution in 1:1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of Cu2+ ion. Explain why?
Ans- FeSO4 solution mixed with (NH4),SO4 solution in 1 : 1 molar ratio forms a double salt, FeS04 (NH4)2SO4-6H2O (Mohr’s salt) which ionizes in the solution to give Fe2+ions. Hence it gives the tests of Fe2+ ions. CuSO4 solution mixed with aqueous ammonia in 1:4 molar ratio forms a complex salt, with the formula [CU(NH3)4]SO4. The complex ion [Cu(NH3)4]2+ does not ionize to give Cu2+ ions. Hence, it does not give the tests of Cu2+ ion.
(NH4 )2SO4 + FeSO4 + 6H2O ⟶ FeSO4. (NH4 )2SO4. 6H2O (Mohr Salt) CuSO4 + 4NH3 + 5H2O ⟶ [Cu(NH3 )4SO4 ].5H2O
9.3 Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Ans- Coordination entity: It constitutes of a central atom/ion bonded to fixed number of ions or molecules by coordinate bonds.
[Ni(NH3 )6 ] 2+,[Fe(CN)6 ] 4+ = cationic complex
[PtCl4 ] 2−,[Ag(CN)2 ] − = anionic complex
[Ni(CO)4 ],[Co(NH3 )4Cl2 ] = neutral complex
Ligand : The ions/molecules bound to central atom/ion in coordination entity are called ligands. Ligands in above examples are CL, NH3, CO Coordination number : This is the number of bond formed by central atom/ion with ligands. For example,
N̈H3,H2Ö, Cl −, −OH.
Ligands are usually polar in nature and possess at least one unshared pair of valence electrons.
Homoleptic : Metal is bound to only one kind of ligands eg Ni in[Ni(CO)4]
Heteroletric Metal is bound to more than one kind of ligandseg Coin [CoCl3(NH3)3]
9.4 What is meant by unidentate, didentate and ambidentate ligands? Give two examples for each.
Ans- Unidentate ligands, such as Cl- and NH3, are molecules or ions that only have one donor atom available to make one coordinate bond with the central metal atom.
A didentate refers to a molecule or ion that has two donor atoms and, as a result, forms two coordinate bonds with the central metal atom.
e.g., ethane-1,2-diamine (H2NCH2CH2NH2), oxalate (C2O42-) ion.
Ambidentate ligands are those which can bind to metal ion through two different donor atoms, e.g.,
NO2– and SCN– ion.
9.5 Specify the oxidation numbers of the metals in the following coordination entities:
(i) [Co(H2O)(CN)(en)2] 2+ (ii) [CoBr2(en)2] + (iii) [PtCl4] 2–
(iv) K3[Fe(CN)6] (v) [Cr(NH3)3Cl3]
Ans – (a) O.N. of Co : x + 0 + (-1) + 2(0)= + 2 or x = + 2+ 1 = + 3
(b) O.N. of Pt : x + 4 (-1) =-2 or x =-2 + 4 = + 2
(c) O.N. of Cr : x + 3(0) + 3(- 1) = 0 or x = + 3
(d) O.N. of Co : x + 2(- 1) + 2(0) = + 1 or x = + 1 + 2 = + 3
(e) O.N. of Fe : x + 6 (- 1) = – 3 or x = – 3 + 6 = + 3
9.6 Using IUPAC norms write the formulas for the following:
(i) Tetrahydroxidozincate(II)
(ii) Potassium tetrachloridopalladate(II)
(iii) Diamminedichloridoplatinum(II)
(iv) Potassium tetracyanidonickelate(II)
(v) Pentaamminenitrito-O-cobalt(III)
(vi) Hexaamminecobalt(III) sulphate
(vii) Potassium tri(oxalato)chromate(III)
(viii) Hexaammineplatinum(IV)
(ix) Tetrabromidocuprate(II)
(x) Pentaamminenitrito-N-cobalt(III)
Ans – (a) [Zn(OH)4]2-
(b) [Pt(NH3)6]4+
(c) K2[PdCl4]
(d) [Cu(Br)4]2-
(e) [CO(NH3)6]2 (SO4)3
(f) K2[Ni(CN)4]
(g) K3 [Cr(OX)3]
(h) [CO(NH3)5ONO]2+
(i) [Pt(NH3)2Cl2]
(j) [CO(NH3)5NO2]2+.
9.7 Using IUPAC norms write the systematic names of the following:
(i) [Co(NH3)6]Cl3 (ii) [Pt(NH3)2Cl(NH2CH3)]Cl (iii) [Ti(H2O)6] 3+
(iv) [Co(NH3)4Cl(NO2)]Cl (v) [Mn(H2O)6] 2+ (vi) [NiCl4] 2–
(vii) [Ni(NH3)6]Cl2 (viii) [Co(en)3] 3+ (ix) [Ni(CO)4]
Ans – (a) hexamminecobalt(III) chloride
(b) tetramminechloriodonitrito-N-cobalt(III) chloride
(c) hexaamminenickel(II) chloride
(d) diamminechlorido (methaneamine) platinum(II) chloride
(e) hexaaquamanganese(II) ion
(f) tetrachloriodonickelate(II) ion
(g) tris(ethane-l, 2-diammine) cobalt(III) ion
(h) hexaaquatitanium(III) ion
(i) tetracarbonylnickel (0).
9.8 List various types of isomerism possible for coordination compounds, giving an example of each.
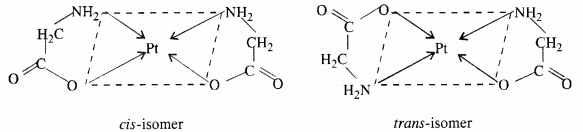
Ans – (i) Geometric isomerism: Heteroleptic complexes frequently exhibit this kind of isomerism. It results from the various conceivable geometric configurations of the ligands. For \example:
when coordination number 4 is present. Since all four places in a tetrahedral geometry are equal, the tetrahedral compounds do not exhibit geometric isomerism. Moving on, there are various options for square planar complexes depending on the composition.
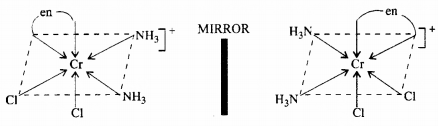
(ii) Optical isomerism: The dextro rotatory isomer, labelled as d-, rotates the plane of polarised light to the right, while the laevo rotatory isomer, designated as l, rotates the plane of polarised light to the left. With the exception of how they behave when exposed to plane polarised light, these optical isomers have identical physical and molecular characteristics.
(iii) Linkage isomerism is a phenomenon that appears in coordination compounds with ambidentate ligands. Complexes containing the thiocyanate ligand, NCS-, which may bind through the nitrogen to create M-N CS or through the sulphur to provide M-SCN, provide a straightforward example. The compound [CO(NH3)5(NO2)]Cl2 exhibits this behaviour in both its red (ONO), in which the nitrite ligand is bound by oxygen, and its yellow (NY), in which it is bound through nitrogen (-NO2).
(iv) The exchange of ligands between cationic and anionic entities of various metal ions present in a complex gives rise to the type of isomerism known as coordination . [Co(NH3)6] [Cr(CN)6], in which the NH3 ligands are coupled to CO3+ and CN, serves as an example.
(v) Ionisation isomerism: This type of isomerism arises when a counter ion replaces a ligand within the coordinator sphere. Thus, complexes that have the same composition, but furnish different ions when dissolved in water are called ionisation isomers. For e.g.,(i) Co(NH3 )5SO4)Br and Co(NH3 )5Br]SO4.
(vi) Solvate isomerism: Solvate isomers differ by whether or not the solvent molecule is directly bonded to the metal ion or merely present as a free solvent molecule in the crystal lattice.
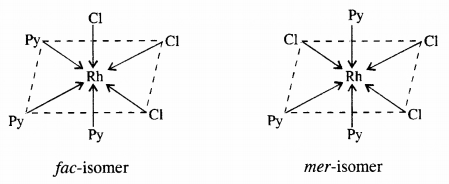
9.9 How many geometrical isomers are possible in the following coordination entities?
(i) [Cr(C2O4)3] 3– (ii) [Co(NH3)3Cl3]
Ans – (i) [Cr(C2O4)3]3- => No geometrical isomers are possible in this coordination entity.
(ii) [Co(NH3)3 Cl3] => Two geometrical isomers are possible (fac and mer) in this coordination entity.
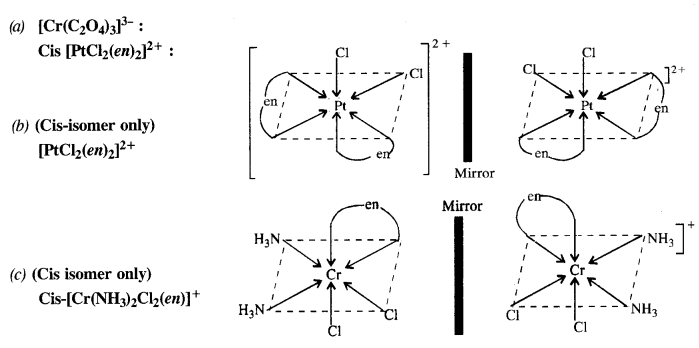
9.10 Draw the structures of optical isomers of:
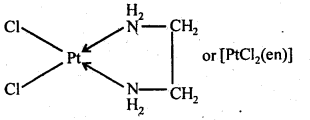
(i) [Cr(C2O4)3] 3– (ii) [PtCl2(en)2] 2+ (iii) [Cr(NH3)2Cl2(en)]
Ans –
9.11 Draw all the isomers (geometrical and optical) of:
(i) [CoCl2(en)2] + (ii) [Co(NH3)Cl(en)2] 2+ (iii) [Co(NH3)2Cl2(en)]+
Ans –
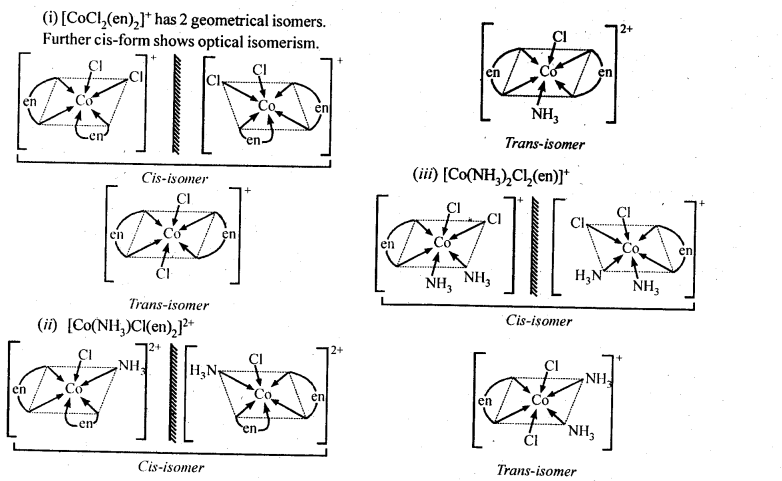
9.12Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers?
Ans – [Pt(NH3)(Br)(Cl)(py)
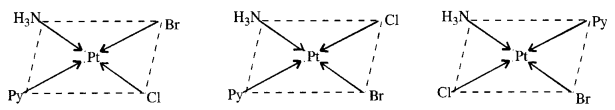
These are obtained by keeping the position of one of the ligand, say NH3 fixed and rotating the positions of others. From the above isomers, none will exhibit optical isomerism. Tetrahedral complexes rarely show optical isomerisation.
9.13 Aqueous copper sulphate solution (blue in colour) gives:
(i) a green precipitate with aqueous potassium fluoride and
(ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Ans –Aqueous solution of copper sulphate which is blue in colour exists as [Cu(H2O)4]SO4 and gives [Cu(H2O)4]2+ in solution. It is a labile complex entity in which the ligands H2O get easily replaced by F– ions of KF and by Cl– ions of KCl
- When KF is added:
[Cu(H2O)4 ] 2+ + 4F − ⟶ +4H2O
- When KCl is added:
[Cu(H2O)4 ] 2+ + 4Cl − ⟶ 4H2O
In both these cases, the weak field ligand water is replaced by the F − and Cl − ions.
9.14 What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
Ans – CuSO4 in aqueous solution is combined with extra aqueous KCN to generate potassium tetra-cyanocuprate (II). Due to the strong ligand properties of CN- ions and the stability of the complex [Cu(CN)4]2-, no copper sulphide precipitate is produced when H2S(g) is passed through the aforesaid solution. CuS precipitate does not develop since there aren’t any Cu2+ ions present.
CuSO4 (s) + 4KCN(aq) ⟶ K2 [Cu(CN)4 ] (aq) + K2SO4 (aq)
i.e., [Cu(H2O)4 ] 2+ + 4CN − ⟶ [Cu(CN)4 ] 2− + 4H2O
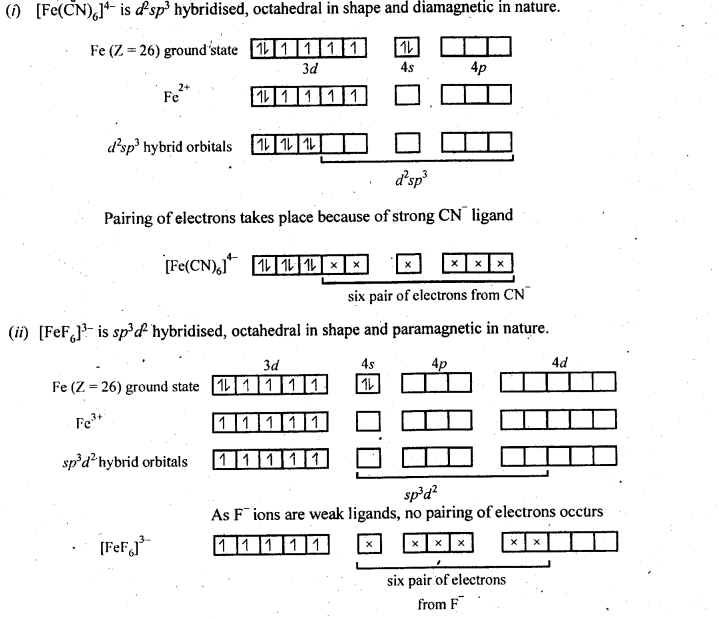
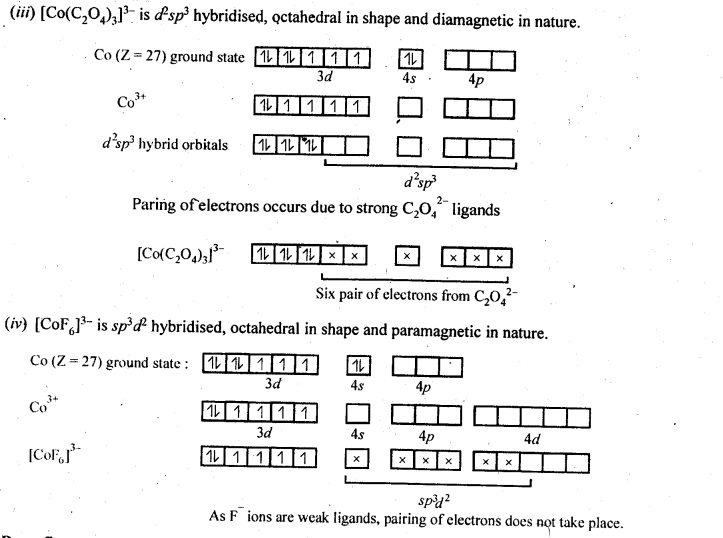
9.15 Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6] 4– (ii) [FeF6] 3–
(iii) [Co(C2O4)3] 3– (iv) [CoF6] 3–
Ans –
9.16 Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Ans – Let us assume that the six ligands are positioned symmetrically
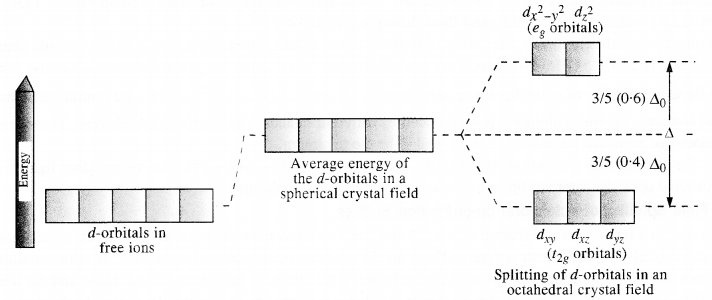
The splitting of the d orbitals in an octahedral field takes place in such a way that dx 2−y 2, dz 2 experience a rise in energy and form the eg level.
9.17 What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Ans – The arrangement of common ligands in an increasing order of their Crystal-Field Splitting Energy (CFSE) values is known as a spectrochemical series. Strong field ligands are located on the right hand side of the series, whereas weak field ligands are present on the light hand side. Additionally, compared to weak field ligands, strong field ligands generate a larger splitting in the d orbitals.
I – <Br -< S 2 – <SCN – <Cl – <N3 – <F – <OH – <C2O4 2 – <H2O – <NCS – <CN – <NH3 – <en – <SO3 2 – <NO2 – <phen – <CO
9.18 What is crystal field splitting energy? How does the magnitude of ∆o decide the actual configuration of d orbitals in a coordination entity?
Ans – In the presence of ligands, the degenerate d-orbitals (in a spherical field environment) break into the two levels, eg and t2g. Crystal field splitting is the splitting of the degenerate orbitals in the presence of ligands, and the crystal field splitting energy is the difference in energy between the two levels (e and t2g). O stands for it. The filling of the electrons occurs after the orbitals have divided. The filling of the electrons occurs in two different ways once one electron (of each type) has filled each of the three t2g orbitals.
After the orbitals have split, the filling of the electrons takes place. After 1 electron (each) has been filled in the three t2g orbitals, the filling of the fourth electron takes place in two ways. It can enter the eg orbital (giving rise to t2g 3 eg 1 like electronic configuration) or the pairing of the electrons can take place in the t2g orbitals.
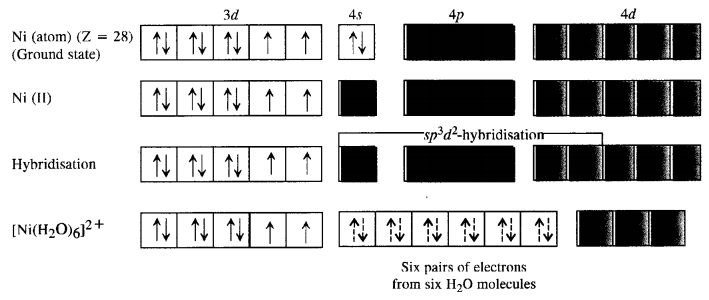
9.19 [Cr(NH3)6] 3+ is paramagnetic while [Ni(CN)4] 2– is diamagnetic. Explain why?
Ans –
![[Cr(NH3)6] 3+ is paramagnetic while [Ni(CN)4] 2– is diamagnetic](https://cbsetoppernotes.com/wp-content/uploads/2022/11/CrNH36-3-is-paramagnetic-while-NiCN4-2–-is-diamagnetic.png)
9.20 A solution of [Ni(H2O)6] 2+ is green but a solution of [Ni(CN)4] 2– is colourless. Explain.
Ans – In [Ni(H20)6]2+, Ni is in + 2 oxidation state and having 3d8 electronic configuration, in which there are two unpaired electrons which do not pair in the presence of the weak H20 ligand. Hence, it is coloured. The d-d transition absorbs red light and the complementary light emitted is green.
9.21 [Fe(CN)6] 4– and [Fe(H2O)6] 2+ are of different colours in dilute solutions. Why?
Ans – Fe is in a + 2 oxidation state with a d6 structure in both complexes. It possesses four unpaired electrons as a result. In the spectrochemical series, the ligands CN- ion and H2O molecules occupy various relative locations. In terms of crystal field splitting energy (0), they differ. They clearly absorb energy from the visible spectrum of light at various wavelengths and frequencies. The transmitted colours and (VIBGYOR) are also varied. This indicates that the complexes’ solutions have various colours.
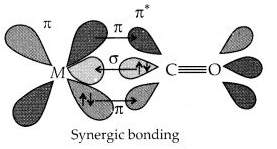
9.22 Discuss the nature of bonding in metal carbonyls.
Ans – In metal carbonyls, the metal-carbon bonds have the characteristics and. When the carbonyl carbon contributes a single pair of electrons to the metal’s open orbital, a bond is created. A bond is created when a pair of electrons from a full metal d orbital are donated into a free anti-bonding orbital. This process is also referred to as back bonding of the carbonyl group. The bond is made stronger by the bond and vice versa.
This metal-ligand interaction subsequently produces a synergistic impact. The link between CO and the metal is strengthened by this synergistic effect.
9.23 Give the oxidation state, d orbital occupation and coordination number of the central metal ion in the following complexes:
(i) K3Co(C2O4)3] (iii)(NH4)2[CoF4]
(ii) cis-[CrCl2(en)2]Cl (iv) [Mn(H2O)6]SO4
Ans – (a) OS = + 3, CN = 6, d-orbital occupation is 3d6 t62ge0g,
(b) OS = + 2, CN = 4, 3d7 (t52ge2g),
(c) OS = + 3, CN = 6, 3d3 (t32g),
(d) OS = + 2, CN = 6, 3d6 (t32ge2g).
9.24 Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number.
Also give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O (iii) CrCl3(py)3 K4[Mn(CN)6]
(ii) [Co(NH3)5Cl- ]Cl2 (iv) Cs[FeCl4]
Ans – (i) IUPAC name : potassium diaquadioxalatochromate (III) hydrate.
O.S. of Cr = + 3 ; 3d3 (t32ge0g) CN = 6 ; shape = octahedral, three unpaired electrons.
Magnetic moment (μ) = n(n+2)−−−−−−−√=3×5−−−−√=15−−√=3⋅87BM
(ii) [Co(NH3)5CIlCl2IUPAC name is pentaamminechloridocobalt (III) chloride Coordination number of Co = 6 Shape is octahedral.
Oxidation state of Co, x + 0 -1 = + 2 .’. x = + 3
Electronic configuration of Co3+ = 3d6 = t62ge°g n=0, μ =0
(iii) IUPAC name : potassiumhexacyanomanganate (II) O.S. of Mn = + 2 ; 3d5 (t52ge0g), CN = 6, shape = octahedral; one unpaired electron.
Magnetic moment (μ) = n(n+2)−−−−−−−√=1×3−−−−√=3–√=1⋅73BM
(iv) IUPAC name : pentaamminechloridocobalt (III) chloride
O.S. of Co = + 3 ; 3d6 (t62ge0g), CN = 6
shape = octahedral; zero unpaired electron. Magnetic moment (μ) = 0
(v) IUPAC name : cesium tetrachloridoferrate (III)
O.S. of Fe = + 3 ; 3d5 (e2t32), CN = 4.
shape = tetrahedral ; five unpaired electrons.
Magnetic moment (μ) = n(n+2)−−−−−−−√=5×7−−−−√=35−−√=5⋅92BM
9.25 What is meant by the stability of a coordination compound in solution? State the factors which govern the stability of complexes..
Ans – The degree of association between the two species involved in the equilibrium state is referred to as the stability of a complex in solution. The association’s stability is expressed quantitatively by the association’s (stability or formation) equilibrium constant’s magnitude. So if we get a reaction of the following kind:
M + 3L ⟷ ML3

The nature of the metal, its oxidation state, and the type of the ligand,
for example, chelating ligands, all affect how stable a complex is
The stability will increase with the ligand’s basic strength.
9.26 What is meant by the chelate effect? Give an example.
Ans – The term “chelate effect” refers to the phenomenon that occurs when a didentate or polydentate ligand contains donor atoms positioned such that, when they coordinate with the central metal ion, a five- or six-membered ring is created. For instance,
For example: Ni(aq) 2+ + 6NH3(aq) ⟷ Ni(aq) 2+ + 3en(aq) ⟷
9.27 Discuss briefly giving an example in each case the role of coordination compounds in:
(i) biological systems (iii) analytical chemistry
(ii) medicinal chemistry and (iv) extraction/metallurgy of metals.
Ans -(i)The role of coordination molecules in biological systems is crucial. Chlorophyll, a pigment involved in photosynthesis, is a coordination molecule of magnesium. The red blood pigment haemoglobin, which serves as an oxygen carrier, is an iron coordination molecule. Cyanocobalamine, often known as vitamin B12 or the anti-pernicious anaemia factor, is a cobalt coordination molecule. The biologically significant molecules that also include coordinated metal ions include enzymes like carboxypeptidase A and carbonic anhydrase (catalysts of biological systems).
(ii)Role of coordination compounds in medicinal chemistry:
Coordination compounds of platinum such as cis-platin is used for inhibiting the growth of tumours.
(iii) The function of coordination compounds in analytical chemistry: During salt analysis, several basic radicals are identified by the colour changes that they undergo in response to various reagents. The coordination molecules or complexes that the basic radicals create with various ligands are what cause these colour shifts.
(iv) The function of coordination compounds in the metallurgy or interaction of metals: Complexes are created during the process of extracting various metals from their ores. For instance, gold and cyanide ions can mix in an aqueous solution to generate [Au (CN)2]. Gold is subsequently recovered from this solution by adding Zn metal.
9.28 How many ions are produced from the complex Co(NH3 )6Cl2 in solution?
(i) 6 (ii) 4 (iii) 3 (iv) 2
Ans – The given complex can be written as [Co(NH3 )6 ]Cl2
Thus, [Co(NH3 )6 ] +2 along with two Cl − ions are produced. So, a total of 3 ions are produced.
9.29 Amongst the following ions which one has the highest magnetic moment value?
(i) [Cr(H2O)6] 3+ (ii) [Fe(H2O)6] 2+ (iii) [Zn(H2O)6] 2+
Ans – The oxidation states of the metals in the complexes along with the electronic configuration are given:
(i) Cr3+ : 3d3 configuration ; unpaired electrons = 3
(ii) Fe2+ : 3d6 configuration ; unpaired electrons = 4
(iii) Zn2+ : 3d10 configuration ; unpaired electrons = 0
The complex (ii) with maximum number of unpaired electrons has the highest magnetic moment. Therefore, (ii) is the correct answer.
9.30 The oxidation number of cobalt in K[Co(CO)4] is
(i) + 1
(ii) + 3
(iii) – 1
(iv) – 3.
Ans – (iii)
We know that CO is a neutral ligand and K carries a charge of +1.
Let the oxidation number of Co = x
Oxidation number =1(+1) + 𝑥 + 4 (0) = 0 𝑥 = −1 Hence, the option (iii) is correct.
9.31 Amongst the following, the most stable complex is
(i) [Fe(H2O)6] 3+ (ii) [Fe(NH3)6] 3+ (iii) [Fe(C2O4)3] 3– (iv) [FeCl6] 3–
Ans – In each of the given complex, Fe is in + 3 oxidation state. As C2042-is didentate chelating ligand, it forms chelate rings and
hence (iii) out of complexes given above is the most stable complex.
9.32 What will be the correct order for the wavelengths of absorption in the visible region for the following:
[Ni(NO2 )6 ] 4–, [Ni(NH3 )6 ] 2+, [Ni(H2O)6 ] 2+ ?
Ans – In all the complexes, the metal ion is the same (Ni2+). The increasing field strengths of the ligands present as per electrochemical series are in the order :
H2O < NH3 < NO–2
The energies absorbed for excitation will be in the order :
[Ni(H2O)6]2+ < [Ni(NH3)6]2+ < [Ni(NO2)6]4-
As E = hc/λ i.e., E ∝ 1/λ; the wavelengths absorbed will be in the opposite order.





