NCERT Exercise Solutions – Chemistry Chapter 5 Surface Chemistry
5.1 Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
Ans– Adsorption is a surface phenomena when substance molecules gather on the surface of a solid or liquid rather than within its interior. The substance that absorbs is referred to as the “adsorbate,” and the substance on the surface of which the adsorption occurs is referred to as adsorbent. Here, the amount of adsorbate on the adsorbent’s surface is more concentrated. In The chemical only concentrates at the surface as a result of adsorption. The thing doesn’t get through the surface to the substance’s or liquid’s main body. As an illustration, when we dip a chalk stick into ink solution, only the surface of the object takes on colour. The chalk stick will reveal itself if we break it inside is white.
5.2 What is the difference between physisorption and chemisorption?
Ans-
| Physisorption | Chemisorption |
|---|---|
| The adsorbate and adsorbent are held by weak van der Waals forces. | The adsorbate and adsorbent are held by forces similar to a chemical bond. |
| Heat of adsorption is of the order of 20 kj/mol. | Heat of adsorption is of the order of 200 kj/mol. |
| It is reversible. | It is irreversible. |
| It decreases with increase in temperature and occurs at lower temperatures. | It increases with temperature and occurs at high temperature. |
| It is not specific in nature, i.e., all gases are adsorbed on all solids to some extent. | It is specific in nature and occurs only when a chemical bond is formed between the adsorbate and adsorbent. |
5.3 Give reason why a finely divided substance is more effective as an adsorbent.
Ans- Adsorption occurs on surfaces. Adsorption is thus inversely proportional to surface area. The surface area of a finely split material is substantial. With an increase in surface area, both physisorption and chemisorption rise. So a substance that has been finely split acts as a good adsorbent.
5.4 What are the factors which influence the adsorption of a gas on a solid?
Ans- The rate of a gas’s adsorption on a solid surface is influenced by a number of variables.
Gas nature: Compared to gases like H2, O2, etc., easily liquefiable gases like NH3, HCl, etc. are adsorbed to a significant extent. This is due to the fact that in easily liquefiable gases, Van der Waal’s forces are stronger.
- The solid’s surface area is The adsorption of a gas onto a solid surface increases with the adsorbent’s surface area.
- Pressure’s result: The reversible process of adsorption results in a drop in pressure. As a result, pressure rises cause an increase in adsorption.
- Adsorption is an exothermic process it is affected by temperature. Consequently, in line with Leehatelie’s concept
5.5 What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Ans- The fluctuation in the amount of gas adsorbed and the related pressure at a specific temperature is represented by the adsorption isotherm. The two adsorption isotherms’ mathematical representations are as follows:

Reundlich adsorption isotherm:
Freundlich adsorption isotherm gives an empirical relationship between the quantity of gas
adsorbed by the unit mass of solid adsorbent and pressure at a specific temperature.
Form the given plot it is clear that at pressure Ps=x/m
reaches the maximum value. Ps is called the saturation pressure.
The following are the primary differences between the two adsorption isotherms:
All types of adsorption can be affected by the Freundlich Adsorption Isotherm, but the Langmuir Adsorption Isotherm primarily affects chemical adsorption or chemisorption.
When the gas pressure is high, the Freundlich adsorption isotherm breaks down, whereas the Langmuir adsorption isotherm works at all pressures.
5.6 What do you understand by activation of adsorbent? How is it achieved?
Ans- An adsorbent is activated by eliminating the gases that it has absorbed and expanding the surface area of the adsorbent to increase its surface area. The adsorption increases with an increase in surface area.
For example, wood charcoal is activated by heating it between 650K and 1330K in vacuum pr air. It expels all the gases absorbed or adsorbed and thus, creates a space for the adsorption of gases.
5.7 What role does adsorption play in heterogeneous catalysis?
Ans- The surfaces of the finely split metals in the transition series are often where heterogeneous catalysis takes place. Because there is a lot of surface area available, the interacting species get physically or chemically adsorbed on the surface. The adsorbed species have the chance to interact with one another to create products that are released or desorbed from the surface to make room for other responding species.
Excellent instances of heterogeneous catalysis include the production of ammonia using iron as a catalyst, the production of H2SO4 by the Contact process, and the hydrogenation of oils using finely split nickel.
5.8 Why is adsorption always exothermic?
Ans- Adsorption is always exothermic. This statement can be explained in two ways.
- Adsorption leads to a decrease in the residual forces on the surface of the adsorbent. This
causes a decrease in the surface energy of the adsorbent. Therefore, adsorption is always
exothermic. - H of adsorption is always negative. When a gas is adsorbed on a solid surface, its movement is restricted leading to a decrease in the entropy of the gas i.e., S is negative. Now for a process to be spontaneous, G should be negative. G = H – T S. Since S is negative H has to be negative to make G negative. Hence, adsorption is always exothermic.
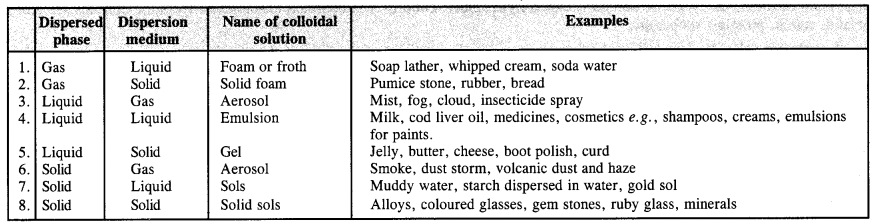
5.9 How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
Ans- The physical characteristics of the dispersed phase and dispersion medium are one criterion for identifying colloids. There are eight different kinds of colloidal systems that can exist depending on the dispersion medium (solid, liquid, or gas) and nature of the dispersed phase.
5.10 Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Ans– Effect of temperature:- Because adsorption is an exothermic process and Le Chatelier’s principle states that a reaction will go in the opposite direction as temperature rises, adsorption reduces as temperature rises.
Effect of pressure:- Pressure causes adsorption to rise at a constant temperature.
5.11 What are lyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated?
Ans-
- Lyophilic sols: Lyophilic sols are colloidal sols made by combining materials like gum, gelatin, starch, etc. with a suitable liquid (dispersion medium). These sols are reversible in nature, meaning that they can be made again by simply mixing the dispersion medium and the dispersion phase and shaking the mixture if two of its components have split apart (for example, by evaporation).
- Lyophobic sols: Substances like metals and their sulphides, etc., do not form colloidal sols when combined with the dispersion medium. Only unique techniques can be used to prepare these colloidal sols. Lyophobic sols are these types of sols. The nature of these sols is irreversible. An illustration. metals in sols.
Now, two factors—the presence of a charge and the survival of colloidal particles—depend on the stability of hydrophilic sols. However, hydrophobic sols are only stable because of the existence of a charge. These are substantially less stable than the earlier, as a result. By adding electrolytes, hydrophobic sols can lose their charge, which causes the particles already present to cluster closer together and precipitate as aggregates.
5.12 What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
Ans-
| Muitimolecular colloids | Macromolecular colloids |
|---|---|
| The particle size is less than that of the colloidal range (< 103 pm). | The particle size falls in the colloidal range (103 to 106 pm). |
| They exist as aggregates of smaller particles. | These are already macro molecular in nature. |
| These are mostly lyophobic colloids. | These are mostly lyophilic colloids. |
Colloids that include more than one kind of atom or tiny molecule, known as multimolecular colloids, have individual particles that are less than 103 pm in size. For instance, gold sol is made up of atom-rich particles of varying sizes. Similar to this, a sulphur sol is made up tiny particles that each contain eight atoms of sulphur (Sg). The van der Waals forces hold the particles in place in these colloids.
Macromolecular colloids: Particles of the dispersed phase that are sufficiently large (macro) to have colloidal dimensions are referred to as this type of colloids. Typically, these are polymers. Starch, cellulose, and proteins are a few examples of macromolecules that are found in nature. Examples of synthetic macromolecules include those found in plastics, nylon, polystyrene, and polythene.
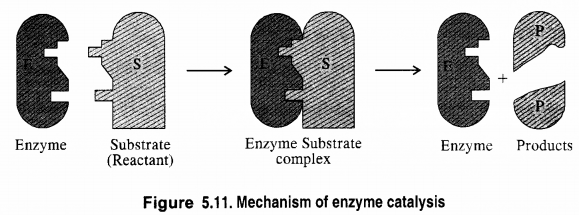
5.13 What are enzymes? Write in brief the mechanism of enzyme catalysis.
Ans- Complex nitrogenous organic molecules known as enzymes serve as biological catalysts and quicken cellular processes. According to the lock and key theory, every enzyme operates on a particular substrate, just as every lock has a unique key.
5.14 How are colloids classified on the basis of
(i) physical states of components
(ii) nature of dispersed phase and
(iii) interaction between dispersed phase and dispersion medium?
Ans– (a) Based on physical states of components.
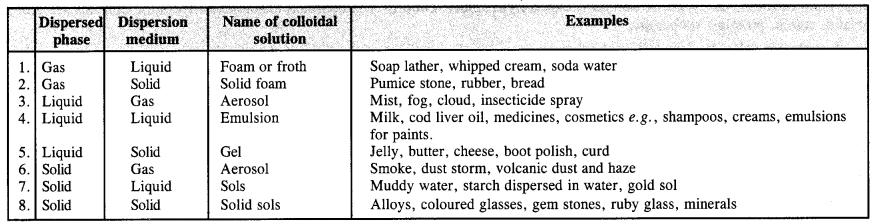
(b) Nature of dispersion medium: Gas, liquid, or solid can all be used as the dispersion medium. There are three different types of colloids or colloidal solutions, depending on their nature.
- Aerosols: The dispersion medium is air or gases.
- Liquid sols: The dispersion medium is a liquid, such as water, alcohol, or benzene.
- Solids serve as the dispersion medium in solid sols.
(c) The interaction of the dispersion medium and the dispersed phase: There are two categories used to classify colloidal solutions. These sols have lyophilicity and lyophobia.
- Lyophilic colloids: Lyophilic colloids are colloidal solutions in which the dispersed phase particles have a strong affinity (or love) for the dispersion medium. When the dispersed phase and the dispersion medium come into direct contact, such solutions are easily created. such as gum sols, gelatin sols, starch sols, etc.
- Lyophobic colloids: Lyophobic colloids are colloidal solutions in which the particles of the dispersed phase harbour antipathy rather than affinities for the dispersion medium. Examples include the solutions of metals like Ag and Au and hydroxides like Al(OH)3 and Fe(OH)3.
5.15 Explain what is observed
(i) when a beam of light is passed through a colloidal sol.
(ii) an electrolyte, NaCl is added to hydrated ferric oxide sol.
(iii) electric current is passed through a colloidal sol?
Ans– (i) Light scattering is seen when a beam of light passes through a colloidal solution. The Tyndall effect is what is meant by this. The beam’s journey through the colloidal solution is made visible by this light scattering.
(ii) Nacl dissociates into Na+ and Cl+ ions when introduced to ferric oxide sol. Positive charges are present in ferric oxide sol particles. They agglomerate as a result when negatively charged Cl-1 ions are present.
(iii)The colloidal particles are positively or negatively charged and are hence charged . A similar and opposing charge is present in the dispersion medium. This neutralises the system as a whole.
The colloidal particles travel in the direction of the electrode with the opposite charge when an electric current is present.
5.16 What are emulsions? What are their different types? Give example of each type.
Ans- These colloidal liquid-liquid systems, or the dispersion of minutely split droplets in another liquid, are liquid-liquid systems. An emulsion is a coarse dispersion of one liquid in the other that results from shaking a combination of two immiscible or partially miscible liquids. Usually, water makes up one of the two liquids. Emulsions come in two different flavours:
Oil dispersed in water (O/W type) and Water dispersed in oil (W/O type)
- Oil-in-water emulsion (O/W type): In this case, the dispersed phase is oil while the dispersion medium is water. Milk is a common example in which liquid fats are dispersed in water. Similarly, if a few drops of nitrobenzene (oil) is added to water, an emulsion results. Vanishing cream is another example of this type.
- Water-in-oil emulsion (W/O type): In this type of emulsions, the dispersed phase is water while the dispersion medium is oil. Butter is an example of water in oil emulsion in which water is dispersed in oil. Cod liver oil and cold cream are the other examples of these emulsions.
5.17 How do demulsifires stabilise emulsion? Name two demulsifiers.
Ans- The process of decomposition of an emulsion into its constituent liquids is called demulsification. Examples of demulsifiers are surfactants, ethylene oxide, etc.
5.18 Action of soap is due to emulsification and micelle formation. Comment.
Ans- A common ingredient in bar soaps is sodium stearate, which is represented by the formula RCOO- Na+. Soap is the sodium or potassium salt of a higher fatty acid. It separates into RCOO- and Na+ ions when dissolved in water. The RCOO- ions, however, are made up of two components: a polar group COO- (also known as the polar ionichead’), which is hydrophilic, and a lengthy hydrocarbon chain R (also known as the non-polar tail’), which is hydrophobic (repells water) (water loving).
As a result, the hydrocarbon chains R remain at the surface and the RCOO- ions are found there with their COO- groups in the water. However, at a crucial micelle concentration, the anions are drawn into the main body of the solution and group together to form a sphere with the COO-part of their hydrocarbon chains pointing outward on the surface. The resultant aggregation is referred to as a “ionic micelle.”
Because the hydrophobic portion of the stearate ions is present in the oil droplet, soap molecules form micelles around it, which has a cleansing effect.
5.19 Give four examples of heterogeneous catalysis.
Ans- (i) Oxidation of sulphur dioxide to form sulphur trioxide. In this reaction, Pt acts as catalyst.
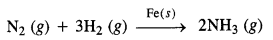
(ii) Formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron.
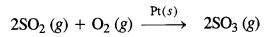
(iii) Oswald’s process: Oxidation of ammonia to nitric oxide in the presence of platinum.
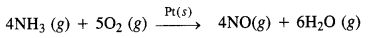
(iv) Hydrogenation of vegetable is in the presence of Ni.
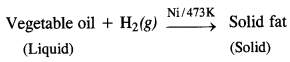
5.20 What do you mean by activity and selectivity of catalysts?
Ans- (a) Activity: The strength of chemisorption has a significant impact on a catalyst’s activity. For the catalyst to function, the reactants must adhere to it fairly strongly. However, adsorption must not be so intense that it renders them immobile. It has been noted that elements in groups 7 – 9 of the periodic table have the highest level of activity.
2H2 + O2 Pt−→ 2H2O
(b) Selectivity: A catalyst’s capacity to produce a certain product during a reaction is referred to as selectivity.
5.21 Describe some features of catalysis by zeolites.
Ans- Zeolites are alumino- silicate materials with minute pores. Zeolites are shape-selective catalysts because of their structure, which resembles a honeycomb. They have a wide-ranging 3D network.
Of silicates that have an Al-O-Si structure due to the replacement of certain silicon atoms with aluminium atoms. Zeolites’ pores and cavity sizes have a huge impact on the reactions that occur inside of them. Zeolites are frequently employed by the petrochemical sector.
5.22 What is shape selective catalysis?
Ans- Shape-selective catalysis refers to the catalytic reaction that depends on the pore structure of the catalyst and the sizes of the reactant and product molecules. Zeolites’ honeycomb-like features make them effective shape-selective catalysts. They are microporous aluminosilicates having an Al-O-Si framework made of a three-dimensional network of silicates in which some silicon atoms have been swapped out for aluminium atoms. The size and form of the reactant and product molecules, as well as the pores and cavities of the zeolites, all affect how reactions occur in zeolites. They can be synthesised for catalytic selectivity or found in nature.
5.23 Explain the following terms:
(i) Electrophoresis
(ii) Coagulation
(iii) Dialysis
(iv) Tyndall effect.
Ans- (i)Electrophoresis: Electrophoresis is the movement of colloidal particles induced by an applied electric field. The cathode attracts positively charged particles, while the anode attracts negatively charged particles. When the particles come into contact with electrodes that have opposite charges, they become neutral and coagulate.
(ii)Coagulation: Coagulation is the process of settling down colloidal particles, or turning a colloid into a precipitate. Electrophoresis, persistent boiling, persistent dialysis, and mutual coagulation are all methods for achieving coagulation.
(iii) Dialysis: Dialysis is the process of extracting dissolved materials from a colloidal solution by diffusion via a membrane. This method is predicated on the idea that, in contrast to colloidal particles, ions and tiny molecules can traverse mammalian membranes.
(iv) Tyndall effect: A column of light appears when a beam of light is allowed to pass through a colloidal solution. The Tyndall effect is what is meant by this. Colloidal particles scatter light in all directions, causing this phenomena.
5.24 Give four uses of emulsions.
Ans- Emulsions have four different applications.
(i) Emulsion production underlies the cleansing effect of soaps.
(ii) The emulsification process is how lipids are broken down in the intestines.
(iii) Disinfectants and antiseptics combine with water to generate emulsions.
(iv) The creation of medications involves the emulsification process.
5.25 What are micelles? Give an example of a micellers system.
Ans- Micelles are substances that function as typical strong electrolytes at low concentrations but turn into colloids at high concentrations due to aggregate formation. They are also known as related colloids, such as detergents and soaps. They have the ability to produce ions and can create micelles with 100 molecules or more.
5.26 Explain the terms with suitable examples:
(i) Alcosol
(ii) Aerosol
(iii) Hydrosol.
Ans–
- Alcosol: It is a colloidal solution in which alcohol is the dispersion medium. For example, colloids which has cellulose nitrate as a dispersed phase and ethyl alcohol as the dispersion medium.
- Aerosol: It is a colloidal solution in which liquid is a dispersed phase and gas is a dispersion medium e.g., fog, mist, cloud, etc.
- Hydrosol: It is a colloidal solution in which solid is a dispersed phase and water is a dispersion, e.g., gold sol, arsenious sulphide sol, ferric oxide sol, etc.
5.27 Comment on the statement that “colloid is not a substance but a state of substance”.
Ans- In a benzene medium, common salt, a typical crystalloid in an aqueous medium, acts as a colloid. Therefore, we can conclude that a colloidal material is not a distinct class of substances. The solute particle behaves as a colloid when its size is between 1 nm and 1000 nm.
As a result, we can conclude that a colloid is not a substance but rather a condition of a substance that depends on the particle size. The transitional condition between a real solution and a suspension is called a colloidal state.


