NCERT Exercise Solutions – Chemistry Chapter 3 Electrochemistry
3.1 Arrange the following metals in the order in which they displace each other from the solution of their salts. Al, Cu, Fe, Mg and Zn.
Ans- Mg, Al, Zn, Fe, Cu, Ag.
3.2 Given the standard electrode potentials, K + /K = –2.93V, Ag+ /Ag = 0.80V, Hg2+/Hg = 0.79V Mg2+/Mg = –2.37 V, Cr3+/Cr = – 0.74V Arrange these metals in their increasing order of reducing power.
Ans- Greater reducing power results from easier oxidation and higher oxidation potential. As a result, A will be the diminishing power in ascending order.
Ag < Hg < Cr < Mg < K.
3.3 Depict the galvanic cell in which the reaction Zn(s)+2Ag+ (aq) →Zn2+(aq)+2Ag(s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Ans- Zn(s) | Zn2+ (aq) || Ag+ (aq) | Ag(s)
(i) Zn electrode (anode) is negatively charged
(ii) Tons are carriers of current in the cell and in the external circuit, current from silver to Zinc.
(iii) The reaction taking place at the anode is given by, Zn(s) -H → Zn2+(aq) + 2e–
3.4 Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd
(ii) Fe2+(aq) + Ag+ (aq) → Fe3+(aq) + Ag(s) Calculate the ∆rG J and equilibrium constant of the reactions.
Ans-

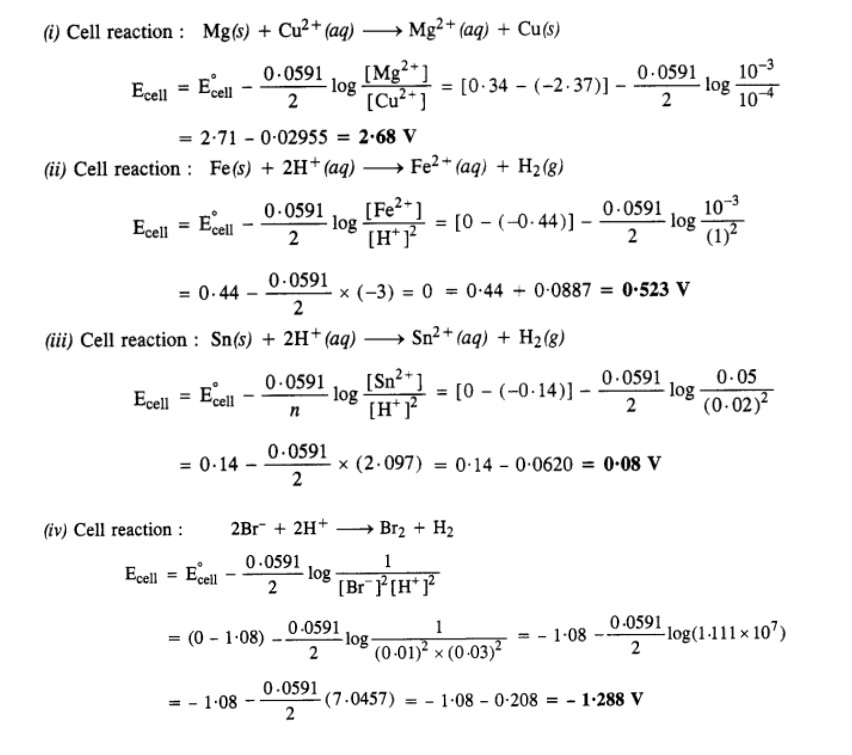
3.5 Write the Nernst equation and emf of the following cells at 298 K:
(i) Mg(s)|Mg2+(0.001M)||Cu2+(0.0001 M)|Cu(s)
(ii) Fe(s)|Fe2+(0.001M)||H+ (1M)|H2 (g)(1bar)| Pt(s)
(iii) Sn(s)|Sn2+(0.050 M)||H+ (0.020 M)|H2 (g) (1 bar)|Pt(s)
(iv) Pt(s)|Br– (0.010 M)|Br2 (l )||H+ (0.030 M)| H2 (g) (1 bar)|Pt(s).
Ans-
3.6 In the button cells widely used in watches and other devices the following reaction takes place:
Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH– (aq)
Determine ∆r G J and E J for the reaction.
Ans-
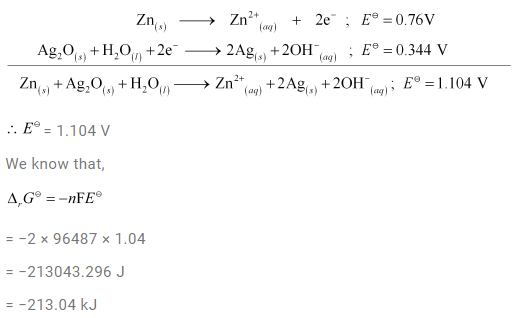
3.7 Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Ans- The area of the cross-section and the conductance of a solution with a length of 1 cm are used to determine a solution’s conductivity. K is a symbol for 1.
For both weak and strong electrolytes, conductivity always drops as concentration rises. This is due to the fact that when concentration lowers, fewer ions per unit volume are required to carry the current in a solution.
The conductance of a volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with an area of cross-section A and a distance of unit length is the molar conductivity of a solution at a particular concentration.
A reduction in concentration causes an increase in molar conductivity. This is so because one mole’s worth of the solution’s entire volume.
3.8 The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm–1. Calculate its molar conductivity.
Ans-
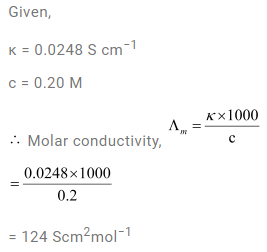
3.9 The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10–3 S cm–1 .
Ans- Cell constant = K × R = 0.146 × 10-3 × 1500 = 0.219 cm-1
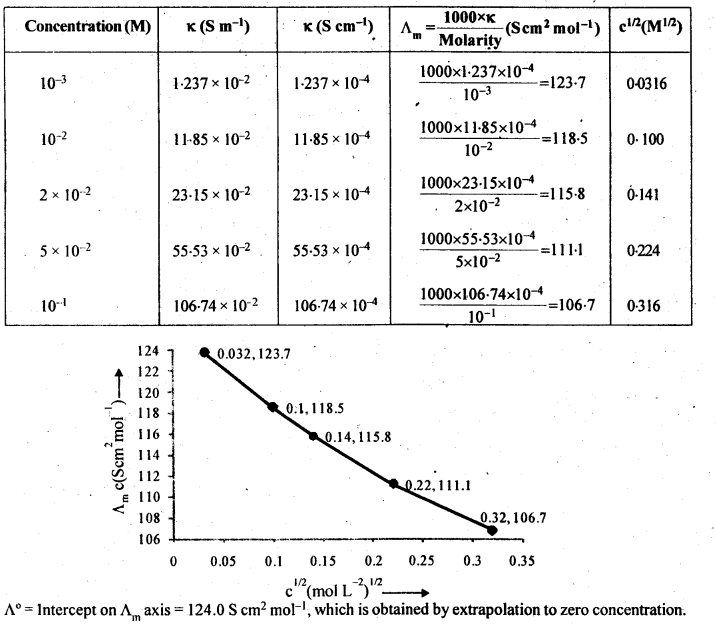
3.10 The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
Concentration/M 0.001 0.010 0.020 0.050 0.100 102 × κ/S m–1 1.237 11.85 23.15 55.53 106.74 Calculate Λm for all concentrations and draw a plot between Λm and c½. Find the value of 0 Λ m .
Ans-
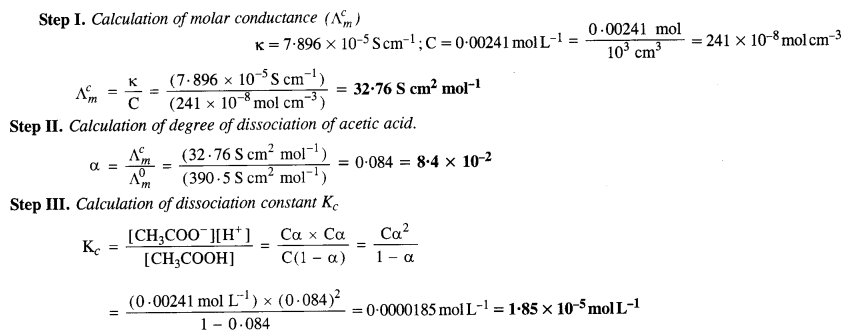
3.11 Conductivity of 0.00241 M acetic acid is 7.896 × 10–5 S cm–1. Calculate its molar conductivity. If 0 Λ m for acetic acid is 390.5 S cm2 mol–1, what is its dissociation constant?
Ans-
3.12 How much charge is required for the following reductions:
(i) 1 mol of Al3+ to Al?
(ii) 1 mol of Cu2+ to Cu?
(iii) 1 mol of MnO4 – to Mn2+
Ans-
(i) The electrode reaction is Al3+ + 3e ——> Al
∴ Quantity of charge required for reduction of 1 mol of Al3+=3F=3 x 96500C=289500C.
(ii) The electrode reaction is Cu2+ + 2e– ——–> Cu
∴ Quantity of charge required for reduction of 1 mol of Cu2+=2F=2 x 96500=193000 C.
(iii) The electrode reaction is Mn04- ———-> Mn2+.
i.e., Mn7+ + 5e–——-> Mn2+.
∴ Quantity of charge required = 5F
=5 x 96500 C=4825000.
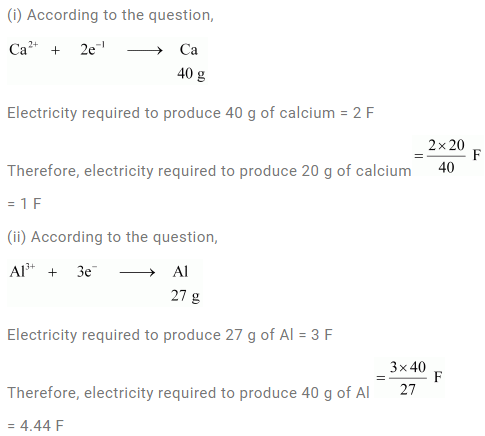
3.13 How much electricity in terms of Faraday is required to produce (i) 20.0 g of Ca from molten CaCl2 ? (ii) 40.0 g of Al from molten Al2O3 ?
Ans-
3.14 How much electricity is required in coulomb for the oxidation of (i) 1 mol of H2O to O2 ? (ii) 1 mol of FeO to Fe2O3 ?
Ans-
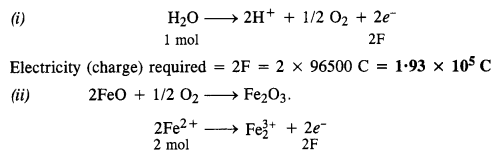
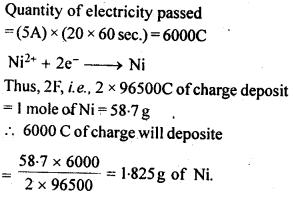
3.15 A solution of Ni(NO3)2 is electrolyzed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Ans-
3.16 Three electrolytic cells A,B,C containing solutions of ZnSO4 , AgNO3 and CuSO4 , respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
Ans-

3.17 Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) Fe3+(aq) and I– (aq)
(ii) Ag+ (aq) and Cu(s)
(iii) Fe3+ (aq) and Br– (aq)
(iv) Ag(s) and Fe 3+ (aq)
(v) Br2 (aq) and Fe2+ (aq).
Ans- A particular reaction can be feasible if e.m.f. of the cell based on the E° values is positive. Keeping this in mind, let us predict the feasibility of the reactions.

3.18 Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3 with platinum electrodes.
(iii) A dilute solution of H2SO4 with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Ans-


