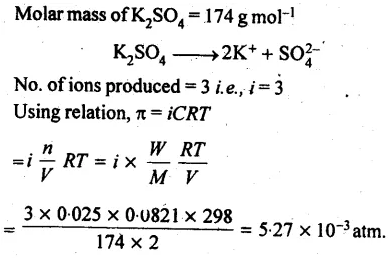NCERT Exercise Solutions – Chemistry Chapter 2 Solutions
2.1 Define the term solution. How many types of solutions are formed? Write briefly about each type with an example.
Ans- A solution is a molecularly homogenous mixture of two or more components. The component of the combination that is present in the smallest amount is referred to as the SOLUTE, and the component that is present in the largest amount as the SOLVENT. For instance, a small amount of water and sugar (solute) dissolved (solvent).
SOLUTE + SOLVENT = SOLUTION
Types of Solution Examples
Gaseous solutions
(b) Gas in a liquid Vaporized water
Vaporized water smoke, N2 gas, and camphor vapour
Liquid solutions
(a) Liquid in liquid, such as water with ethanol dissolved in it.
(b) Solid in a liquid Saline water, sugar dissolved in it, etc.
Solid solutions
(a) Gas in solid Hydrogen in palladium solution
(b) Liquid in solid amalgams, such as Na-Hg
2.2 Give an example of a solid solution in which the solute is a gas.
Ans- For example: tungsten carbide, where tungsten atoms are arranged in a face centred cubic pattern with carbon atoms in octahedral holes.
2.3 Define the following terms:
(i) Mole fraction (ii) Molality
(iii) Molarity (iv) Mass percentage.
Ans- (i) Mole fraction is the ratio of the amount of one component, expressed in moles, to the total amount of solute and solvent present in the solution.
The mole fraction is calculated as follows: moles of the component divided by the sum of all component moles.
The symbol for it is x.
In a binary solution, where nA is the number of moles of solute and nB is the number of moles of solvent, the mole fraction of the solute is given as:
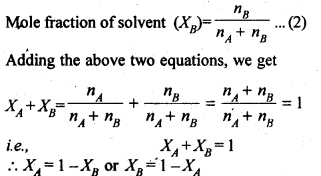
(ii) Molality (m) is defined as the number of moles of the solute per kilogram of the solvent. It is expressed as:
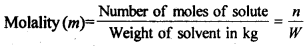
(iii) Molarity is the measure of how many moles of a solute are dissolved in one litre of a solution.
M is a representative of it.

(iv) Mass percentage: It is the amount of solute in grams present in 100g of solution.
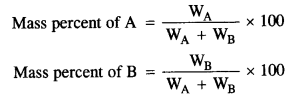
2.4 Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 1.504 g mL–1?
Ans- Nitric acid that has been concentrated for use in lab work has a mass concentration of 68% in an aqueous solution. This indicates that in 100 g of the solution, 68 g of nitric acid have been dissolved.
Molar mass of nitric acid (HNO3) = 1 × 1 + 1 × 14 + 3 × 16 = 63 g mol – 1
Then, number of moles of HNO3 = 68 / 63 mol = 1.08 mol
Also density = 1.504g/mL-1 (given)
Therefore from the formula density = mass / volume, we get
Volume of solution = 1000/1.504 = 66.49 mL
Therefore molarity of nitric acid = (1.08/66.49) x 1000 = 16.24 M
2.5 A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL–1, then what shall be the molarity of the solution?
Ans-

2.6 How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
Ans- Let the amount of Na2CO3 in the mixture be x g.
Then, the amount of NaHCO3 in the mixture is (1 − x) g.
Molar mass of Na2CO3 = 2 × 23 + 1 × 12 + 3 × 16
= 106 g mol−1
Now moles of Na2CO3 = x / 106
& moles of NaHCO3 = 1-x / 84
Now according to question , number of moles of Na2Co3 = number of moles of NaHCO3
Therefore x / 106 = 1-x / 84
84x = 106-106x
84x +106x = 106
190x = 106
Or
x = 106 / 190 = 0.558
Therefore moles of Na2Co3 = 0.558 / 106 = 0.00526
&
moles of NaHCO3 = 1 – 0.558 / 84 = 0.0053
Now Hcl reacts with Na2Co3 & NaHCO3 as follows:
Na2Co3 + 2Hcl 2Nacl + H2o + CO2
NaHCO3 + Hcl Nacl + H2o + CO2
From the above reactions, 1 mol of Na2Co3 will react with 2 mol of Hcl
Therefore 0.00526 mol of Na2Co3 will react with 2 x 0.00526 mol of Hcl & similarly 0.00526 mol of NaHCO3 will react with 0.00526 mol of Hcl
Total moles of Hcl required to react with mixture of NaHCO3 & Na2Co3
= 2 X 0.00526 + 0.00526 =0.01578 mol
Also according to question 0.1 mol of 0.1 M Hcl is present in 1000 ml
2.7 A solution is obtained by mixing 300 g of 25% solution and 400 g of 40% solution by mass. Calculate the mass percentage of the resulting solution.
Ans- According to the above information,
the solute in 300g of a 25% solution is equal to (300 x 25)/100, or 75g,
while the solute in 400g of a 40% solution is equal to (400 x 40)/100, or 160g.
Total solute = 75 + 160 = 235g
Total solution is as stated in the question: 300 + 400 = 700g
Final solution solute content = (235 x 100) / 700 = 33.57%
Percentage of water in the final solution = 100 – 33.5 = 65.43%
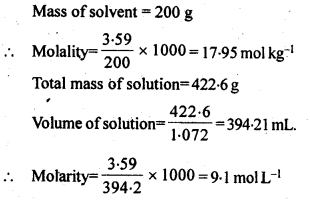
2.8 An antifreeze solution is prepared from 222.6 g of ethylene glycol (C2H6O2 ) and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g mL–1, then what shall be the molarity of the solution?
2.9 A sample of drinking water was found to be severely contaminated with chloroform (CHCl3) supposed to be a carcinogen. The level of contamination was 15 ppm (by mass):
(i) express this in percent by mass
(ii) determine the molality of chloroform in the water sample.
Ans-
1) 15 ppm means : 15 parts per million(106) of the solutions
So, Percent by mass = (mass of chloroform / total mass) x 100
= (15 / 106) x 100
= 1.5 x 10-3%
2) Molality Mass of chloroform = 15 g
Molar mass of chloroform (CHCl3) = 1 × 12 + 1 × 1 + 3 × 35.5
= 119.5 g mol -1
Moles of chloroform = 15 / 119.5 = 0.1255 mol
Mass of water = 106
Therefore molality = (moles of chloroform / mass of water ) x 1000
= (0.1255 / 106) x 1000
= 1.255 x 10-4 m
2.10 What role does the molecular interaction play in a solution of alcohol and water?
Ans- Although the solubility of alcohols declines with increasing molecular weight, the lowest members of the family are very soluble in water. The creation of hydrogen bonds between alcohol molecules and water molecules is the cause of the lower alcohols’ solubility in water.
However, alcohol and water molecules in the solution also have hydrogen bonds, albeit much weaker than those of alcohol and water. As a result, attractive force strength tends to decline, and the solution deviates positively from Raoult’s Law. This will cause the solution’s boiling point to decrease and its vapour pressure to rise.
2.11 Why do gases always tend to be less soluble in liquids as the temperature is raised?
Ans- Gases dissolve in water, which results in the release of heat energy, making the process exothermic. According to Lechatlier’s Principle, the equilibrium shifts backwards as the temperature rises, making gases less soluble in liquids.
2.12 State Henry’s law and mention some important applications.
Ans- According to Henry’s law, the mole fraction of the gas in the solution is directly related to the partial pressure of the gas in the vapour phase. Henry’s law may be written as follows if p is the partial pressure of the gas in the vapour phase and x is the gas’s mole fraction:
p = KH x
Where,
KH is Henry’s law constant
Some important applications of Henry’s law are mentioned below.
(i)To increase the solubility of CO2 in soft drinks and soda water, bottles are sealed under high pressure.
(ii) Henry’s law indicates that as pressure rises, gases become more soluble. As a result, when a scuba diver goes deep underwater, the increasing sea pressure causes a significant quantity of the nitrogen in the air to dissolve in his blood. When he returns to the surface, the solubility of nitrogen again drops, the dissolved gas is liberated, and nitrogen bubbles start to develop in the blood. Bends, often known as “decompression sickness,” is a medical disease caused by the occlusion of capillaries.
2.13 The partial pressure of ethane over a solution containing 6.56 × 10–3 g of ethane is 1 bar. If the solution contains 5.00 × 10–2 g of ethane, then what shall be the partial pressure of the gas?
Ans- According to Henry’s law
.m = k x p
Substituting the given values in the above equation.
We get
6.56 x10-3 = k x 1
Or
k = 6.56 x10-3
Now when m = 5 x 10-2,
Then again substituting the given values in Henry’s law equation, we get
5 x 10-2 = 6.56 x10-3 x p
Or
p = 7.62 bar
2.14 What is meant by positive and negative deviations from Raoult’s law and how is the sign of ∆mixH related to positive and negative deviations from Raoult’s law?
Ans- According to Raoult’s law, the mole fraction of the solvent is exactly proportional to the vapour pressure of a solution containing a nonvolatile solute at a given temperature.
Positive and negative departures from ideal behavior are demonstrated through non-ideal solutions.
Positive deviations from Raoult’s law in imperfect solutions Take into account a binary solution with the components A & B. If the A-B interactions in the two liquids that make up the solution are weaker than the A-A & B-B interactions in those two liquids, then the tendency of A & B type molecules to escape from the solution is greater than it is from pure liquids.
As a result ,each component of solution has a partial vapour pressure greater than expected on the basis of Raoult’s law.This is called positive deviations from Raoult’s law,i.e PA> PA °xA & PB >PB°xB
ΔmixH is positive because energy is required to break A-A & B-B attractive forces. Hence endothermic process.
Non ideal solutions showing Negative deviations from Raoult’s law- in such solutions, the A-B interactions are stronger than A-A & B-B interactions .Due to stronger A-B interactions ,the escaping tendency of A & B types of molecules from the solution becomes less than from pure liquids. Consequently, each component of the solution has a partial vapour pressure less than expected on the basis of Raoults law. This is called negative deviations form Raoults law, i.e PA< PA °xA & PB B°xB.
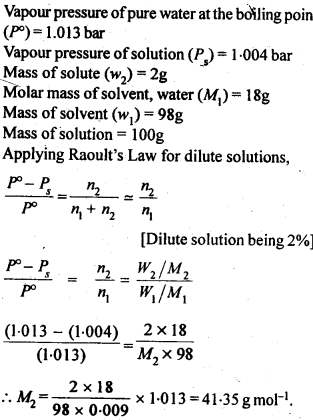
2.15 An aqueous solution of 2% non-volatile solute exerts a pressure of 1.004 bar at the normal boiling point of the solvent. What is the molar mass of the solute?
Ans-
2.16 Heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35 g of octane?
Ans-
: Vapour pressure of heptane p10 = 105.2 kPa
Vapour pressure of octane p20= 46.8 kPa
As we know that, Molar mass of heptane (C7H16) = 7 × 12 + 16 × 1 = 100 g mol -1
∴ Number of moles of heptane = 26/100 mol = 0.26 mol
Molar mass of octane (C8H18) = 8 × 12 + 18 × 1 = 114 g mol -1
∴ Number of moles of octane = 35/114 mol = 0.31 mol
Mole fraction of heptane, x1 = 0.26 / 0.26 + 0.31 = 0.456
And, mole fraction of octane, x2 = 1 – 0.456 = 0.544
Now, partial pressure of heptane, p1 = x2 p20 = 0.456 × 105.2 = 47.97 kPa
Partial pressure of octane,p2 = x2 p20 = 0.544 × 46.8 = 25.46 kPa
Hence, vapour pressure of solution, ptotal = p1 + p2 = 47.97 + 25.46
= 73.43 kPa
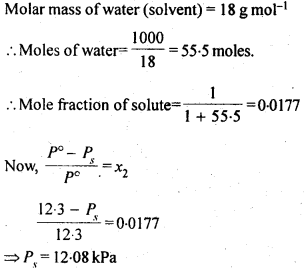
2.17 The vapour pressure of water is 12.3 kPa at 300 K. Calculate vapour pressure of 1 molal solution of a non-volatile solute in it.
Ans- 1 mole of the solute in 1000g of the solvent is referred to as a molal solution.
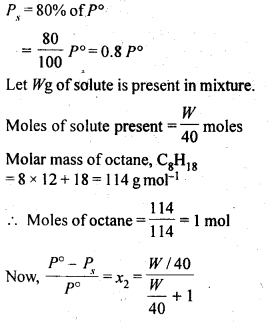
2.18 Calculate the mass of a non-volatile solute (molar mass 40 g mol–1) which should be dissolved in 114 g octane to reduce its vapour pressure to 80%.
Ans- According to Raoult’s Law,
Let the vapour pressure of pure octane be p10.
Then, the vapour pressure of the octane after dissolving the non-volatile solute is 80/100 p10 = 0.8 p10
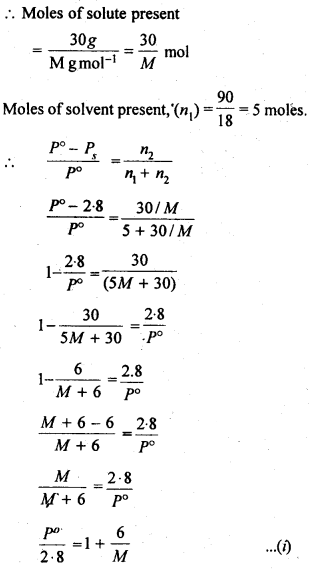
2.19 A solution containing 30 g of non-volatile solute exactly in 90 g of water has a vapour pressure of 2.8 kPa at 298 K. Further, 18 g of water is then added to the solution and the new vapour pressure becomes 2.9 kPa at 298 K. Calculate:
(i) molar mass of the solute
(ii) vapour pressure of water at 298 K.
Ans- Let, the molar mass of the solute be M g mol -1
Now, the no. of moles of solvent (water),n1 = 90g / 18g mol-1
2.20 A 5% solution (by mass) of cane sugar in water has freezing point of 271K. Calculate the freezing point of 5% glucose in water if freezing point of pure water is 273.15 K.
Ans- In case of cane sugar:
ΔTf = (273.15 – 271) K = 2.15 K
Molar mass of sugar (C12H22O11) = 12 × 12 + 22 × 1 + 11 × 16 = 342 g mol – 1
5% solution (by mass) of cane sugar in water means 5 g of cane sugar is present in (100 – 5)g = 95 g of water.
Now, number of moles of cane sugar =5/342 mol
= 0.0146 mol
Therefore, molality of the solution,m =0.0146mol / 0.095kg
= 0.1537 kg mol – 1
Now applying the relation,
ΔTf = Kf × m
⇒ Kf = ΔTf / m
⇒ 2.15K / 0.1537 kg mol-1
= 13.99 K kg mol-1
Molar of glucose (C6H12O6) = 6 × 12 + 12 × 1 + 6 × 16 = 180 g mol – 1
5% glucose in water means 5 g of glucose is present in (100 – 5) g = 95 g of water.
∴ Number of moles of glucose = 5/180 mol
= 0.0278 mol
Therefore, molality of the solution, m =0.0278 mol / 0.095 kg
= 0.2926 mol kg – 1
Applying the relation,
ΔTf = Kf × m
= 13.99 K kg mol – 1 × 0.2926 mol kg – 1
= 4.09 K (approximately)
Hence, the freezing point of 5% glucose solution is (273.15 – 4.09) K= 269.06 K.
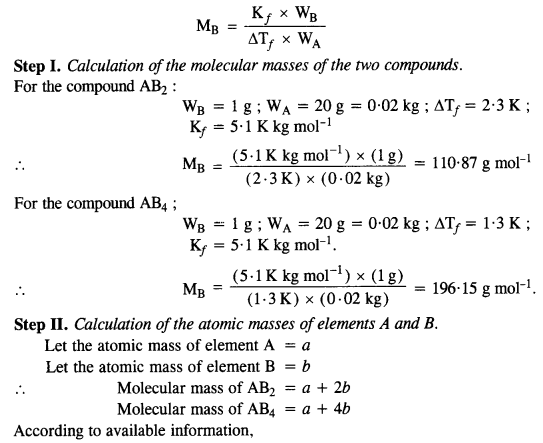
2.21 Two elements A and B form compounds having formula AB2 and AB4 . When dissolved in 20 g of benzene (C6H6 ), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol–1. Calculate atomic masses of A and B.
Ans- As We know that:
2.22 At 300 K, 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar. If the osmotic pressure of the solution is 1.52 bars at the same temperature, what would be its concentration?
Ans- Here we have given
π1= 4.98
π2 = 1.52
C1 = 36/180
C2 = ? (we have to find)

2nd case : 4.98 = 36/180RT ————————1
1.52 = c2RT ————————2
Now dividing equation 2 by 1 ,we get
(c2 x 180) / 36 = 1.52 / 4.98
or
c2 = 0.061
2.23 Suggest the most important type of intermolecular attractive interaction in the following pairs.
(i) n-hexane and n-octane (ii) I 2 and CCl4
(iii) NaClO4 and water (iv) methanol and acetone
(v) acetonitrile (CH3CN) and acetone (C3H6O).
Ans- (i) Van der Wall’s forces of attraction.
(ii) Van der Wall’s forces of attraction.
(iii) Ion-diople interaction.
(iv) Dipole-dipole interaction.
(v) Dipole-dipole interaction.
2.24 Based on solute-solvent interactions, arrange the following in order of increasing solubility in n-octane and explain.
Cyclohexane, KCl, CH3OH, CH3CN.
Ans- A non-polar solvent, n-octane. Because of this, a non-polar solute is more soluble in n-octane than a polar solute.
The order of increasing polarity is:
Cyclohexane < CH3CN < CH3OH < KCl
Therefore, the order of increasing solubility is:
KCl < CH3OH < CH3CN < Cyclohexane
2.25 Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water?
(i) phenol (ii) toluene (iii) formic acid
(iv) ethylene glycol (v) chloroform (vi) pentanol.
Ans- (i) The polar group OH and non-polar group C6H5 are both present in phenol (C6H5OH). So, phenol is only somewhat soluble in water.
(ii) There are no polar groups in toluene (C6H5CH3). Toluene cannot dissolve in water, thus.
(iii) Formic acid (HCOOH) has the polar group −OH and can form H-bond with water. Thus, formic acid is highly soluble in water.
(iv) Ethylene glycol has polar −OH group and can form H−bond. Thus, it is highly soluble in water.
(v) Chloroform is insoluble in water.
(vi) Pentanol (C5H11OH) has polar −OH group, but it also contains a very bulky non-polar −C5H11 group. Thus, pentanol is partially soluble in water.
2.26 If the density of some lake water is 1.25g mL–1 and contains 92 g of Na+ ions per kg of water, calculate the molarity of Na+ ions in the lake.
Ans- We know molality = moles of solute / mass of solvent in kg
Now mass of Na+ ions = 92 g (given)
Moles of Na+ ions = 92 / 23 = 4
And mass of water = 1kg
Therefore molality = 4/1 = 4m
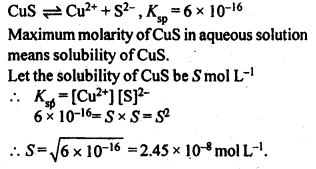
2.27 If the solubility product of CuS is 6 × 10–16, calculate the maximum molarity of CuS in aqueous solution.
Ans-
2.28 Calculate the mass percentage of aspirin (C9H8O4 ) in acetonitrile (CH3CN) when 6.5 g of C9H8O4 is dissolved in 450 g of CH3CN.
Ans- Mass of aspirin = 6.5 g
Mass of acetonitrile = 450 g
Then, total mass of the solution = (6.5 + 450) g = 456.5 g
Therefore, mass percentage of C9H8O4 = 6.5456.5 × 100%
= 1.424%
2.29 Nalorphene (C19H21NO3 ), similar to morphine, is used to combat withdrawal symptoms in narcotic users. Dose of nalorphene generally given is 1.5 mg. Calculate the mass of 1.5 × 10–3 m aqueous solution required for the above dose.
Ans- Molecular mass of nalorphene (C19H21NO3),
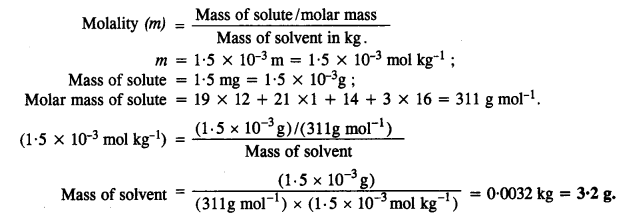
2.30 Calculate the amount of benzoic acid (C6H5COOH) required for preparing 250 mL of 0.15 M solution in methanol.
Ans- We know Molarity = moles of the solute / volume of solution
Putting the given values in above equation,we get
0.15 = (mole of benzoic acid / 250) x 1000
Or
moles of benzoic acid = (0.15 x 250) / 1000
= 0.0375 mol of benzoic acid
Also molecular mass of benzoic acid (C6H5COOH)
= 7 × 12 + 6 × 1 + 2 × 16
= 122 g/mol
Therefore amount of benzoic acid = 0.0375 x 122 = 4.575 g
2.31 The depression in freezing point of water observed for the same amount of acetic acid, trichloroacetic acid and trifluoroacetic acid increases in the order given above. Explain briefly.
Ans-
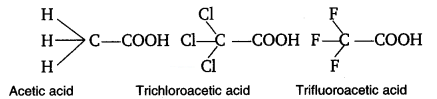
The degree of ionisation, which is dependent on the acid strength, can be used to explain the above trend in the decrease in the freezing point of water for the same amount of the supplied chemicals. More acidic than trichloroacetic acid, which is itself more acidic than acetic acid, is trifluroacetic acid.
Therefore the degree of ionization of these acids will decrease in the following order:
Trifluoroactic acid > trichloroacetic acid > acetic acid
Now greater the degree of ionization ,greater will be the depression of freezing point.
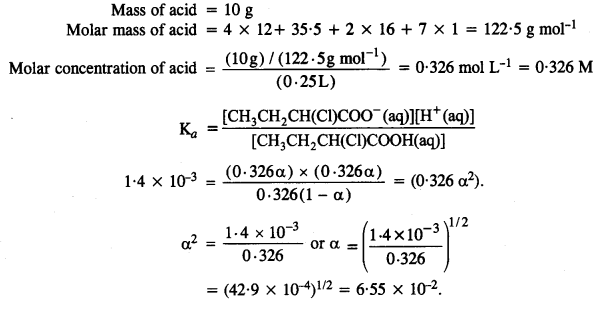
2.32 Calculate the depression in the freezing point of water when 10 g of CH3CH2CHClCOOH is added to 250 g of water.
Ans-
Mass of solute (CH3CH2CHClCOOH) = 10 g
Molar mass of
CH3CH2CHClC00H = 4 × 12 + 7 × 1 + 1 × 35.5 + 2 × 16 = 48 + 7 + 35.5 + 32
= 122.5 g mol-1
2.33 19.5 g of CH2 FCOOH is dissolved in 500 g of water. The depression in the freezing point of water observed is 1.00 C. Calculate the van’t Hoff factor and dissociation constant of fluoroacetic acid.
Ans-
Calculation of Van’t Hoff factor (i)
Given, w1 = 500 g = 0.5 kg, w2 = 19.5 g, Kf = 1.86 K kg mol-1, ΔTf = 1 K
Molar mass of CH2FCOOH (M2)
= 2 × 12 + 3 × 1 + 1 × 19 + 2 × 16
= 24 + 3 + 19 + 32
= 78 g mol-1
ΔTf = i Kf m
=1.86 x 0.50 = 0.93K
Van’t hoff factor = observed freezing point depression / calculated freezing point depression
= 1 / 0.93 = 1.0753
Let α be the degree of dissociation of CH2FCOOH
i = α(1+α) / α = 1 +α = 1.0753
Therefore α = 1.0753- 1
= 0.0753
Now the Value of Ka is given as:
Ka = [CH2FCOO–][H+] / CH2FCOOH
= (Cα x Cα) / (C (1-α))
= Cα2 / (1-α)
Ka = 0.5 X (0.0753)2 / (1-0.0753)
= 0.5 X 0.00567 / 0.09247
= 0.00307 (approx.)
= 3 X 10-3
2.34 Vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapour pressure of water at 293 K when 25 g of glucose is dissolved in 450 g of water.
Ans- According to Raoult’s Law,
Vapour pressure of water, p1° = 17.535 mm of Hg
Mass of glucose, w2 = 25 g
Mass of water, w1 = 450 g
We know that,
Molar mass of glucose (C6H12O6),
M2 = 6 × 12 + 12 × 1 + 6 × 16 = 180 g mol – 1
Molar mass of water, M1 = 18 g mol – 1
Then, number of moles of glucose, n1 = 25/180 = 0.139 mol
And, number of moles of water, n2 =450/18 = 25 mol
Now, we know that,
(p1° – p°) / p1° = n1 / n2 + n1
⇒ 17.535 – p° / 17.535 = 0.139 / (0.139+25)
⇒ 17.535 – p1 = 0.097
⇒ p1 = 17.44 mm of Hg
Hence, the vapour pressure of water is 17.44 mm of Hg.
2.35 Henry’s law constant for the molality of methane in benzene at 298 K is 4.27 × 105 mm Hg. Calculate the solubility of methane in benzene at 298 K under 760 mm Hg.
Ans- Here, p = 760 mm Hg, KH = 4.27 × 105 mm Hg (at 298 K)
According to Henry’s law, p = KHχ
χ = pkH
= 760 mmHg4.27×105 mmHg
= 177.99 × 10-5
= 178 × 10-5
Hence, the mole fraction of methane in benzene is 178 × 10-5.
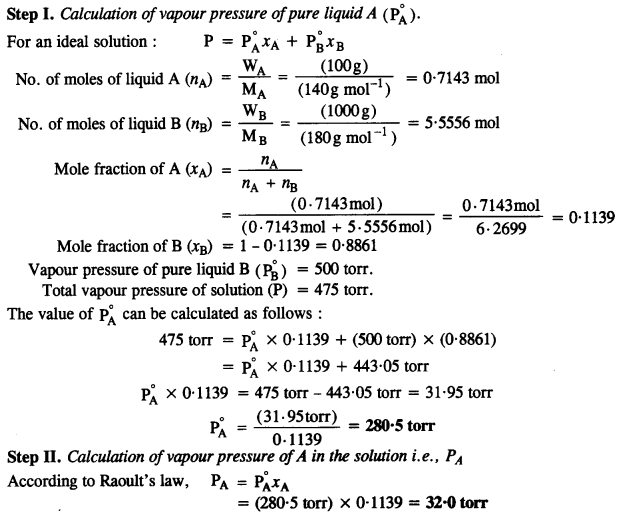
2.36 100 g of liquid A (molar mass 140 g mol–1) was dissolved in 1000 g of liquid B (molar mass 180 g mol–1). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 Torr.
2.37 Vapour pressures of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot ptotal, pchloroform, and pacetone as a function of xacetone. The experimental data observed for different compositions of mixture is:
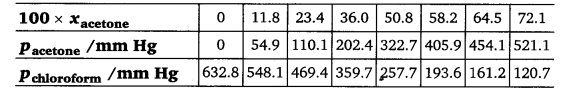
Ans- From the question, we have the following data
| 100 ×xacetone | 0 | 11.8 | 23.4 | 36.0 | 50.8 | 58.2 | 64.5 | 72.1 |
| pacetone /mm Hg | 0 | 54.9 | 110.1 | 202.4 | 322.7 | 405.9 | 454.1 | 521.1 |
| pchloroform/mm Hg | 632.8 | 548.1 | 469.4 | 359.7 | 257.7 | 193.6 | 161.2 | 120.7 |
| ptotal(mm Hg) | 632.8 | 603.0 | 579.5 | 562.1 | 580.4 | 599.5 | 615.3 | 641.8 |
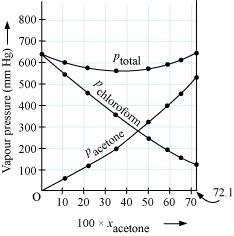
It can be observed from the graph that the plot for the ptotalof the solution curves downwards. Therefore, the solution shows negative deviation from the ideal behaviour.
2.38 Benzene and toluene form ideal solution over the entire range of composition. The vapour pressure of pure benzene and toluene at 300 K are 50.71 mm Hg and 32.06 mm Hg respectively. Calculate the mole fraction of benzene in vapour phase if 80 g of benzene is mixed with 100 g of toluene.
Ans- Molar mass of benzene(C6H6) = 6 X 12 + 6 X 1 = 78 g/mol
Molar mass of toluene = 7 x 12 + 8 x 1 = 92 g/mol
2.39 The air is a mixture of a number of gases. The major components are oxygen and nitrogen with approximate proportion of 20% is to 79% by volume at 298 K. The water is in equilibrium with air at a pressure of 10 atm. At 298 K if the Henry’s law constants for oxygen and nitrogen at 298 K are 3.30 × 107 mm and 6.51 × 107 mm respectively, calculate the composition of these gases in water.
Ans- Percentage of oxygen (O2) in air = 20 %
Percentage of nitrogen (N2) in air = 79%
Also, it is given that water is in equilibrium with air at a total pressure of 10 atm, that is, (10 × 760) mm Hg = 7600 mm Hg
Therefore, Partial pressure of oxygen, po2 = 20/100 *7600
=1520 mm Hg
Partial pressure of nitrogen,pN2 = 79/100 *7600
= 6004 mmHg
Now, according to Henry’s law:
p = KH.x
For oxygen:
po2 = KH. xO2
⇒xO2 = po2 / KH
= 1520 / 3.30 X 107
= 4.61 10-5
For nitrogen:
pN2 = KH.xN2
⇒xN2 = pN2 / KH
= 6004 / 6.51 x 107
= 9.22 x 10-5
Hence, the mole fractions of oxygen and nitrogen in water are 4.61 ×10 – 5 and 9.22 × 10 – 5 respectively
2.40 Determine the amount of CaCl2 (i = 2.47) dissolved in 2.5 litre of water such that its osmotic pressure is 0.75 atm at 27° C.
Ans- We know that,
π = i RT
⇒ π = i RT
⇒ w =
Given,
π = 0.75 atm
V = 2.5L
i = 2.47
T = (27 + 273)K = 300K
R = 0.0821 L atm K-1mol–
Molar mass of CaCl2(M) = 1 × 40 + 2 × 35.5 = 111 g mol -1
Therefore, w = 0.75×111×2.52.47×0.0821×300 = 3.42g
Hence the required amount of CaCl2 is3.42g
2.41 Determine the osmotic pressure of a solution prepared by dissolving 25 mg of K2SO4 in 2 litre of water at 25° C, assuming that it is completely dissociated.