NCERT Exercise Solutions – Chemistry Chapter-11 Alcohols, Phenols and Ethers
11.1 Write IUPAC names of the following compounds:
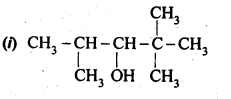
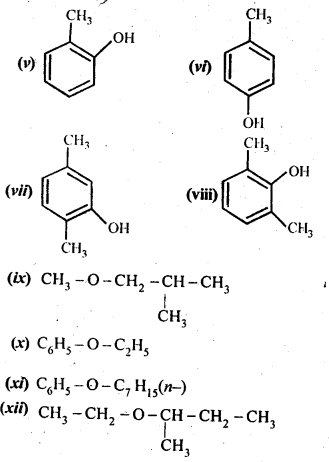
Ans – (i) 2,2,4-Trimethylpentan-3-ol
(ii) 5-Ethylheptane-2,4-dioI
(iii) Butane-2,3-diol
(iv) Propane-1,2,3-triol
(v) 2-Methylphenol
(vi) 4-Methylphenol
(vii) 2,5-DimethylphenoI
(viii) 2,6-Dimethylphenol
(ix) 1-Methoxy-2-methylpropane
(x) Ethoxybenzene
(xi) 1-Phenoxyheptane
(xii) 2-Ethoxybutane
11.2 Write structures of the compounds whose IUPAC names are as follows:
(i) 2-Methylbutan-2-ol
(ii) 1-Phenylpropan-2-ol
(iii) 3,5-Dimethylhexane –1, 3, 5-triol
(iv) 2,3 – Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan-3-ol
(ix) Cyclopent-3-en-1-ol
(x) 4-Chloro-3-ethylbutan-1-ol.
Ans –
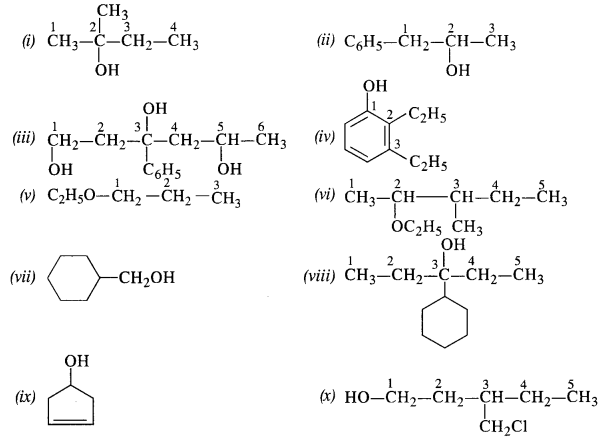
11.3 (i) Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names.
(ii) Classify the isomers of alcohols in question 11.3 (i) as primary, secondary and tertiary alcohols.
Ans – (i) The structures of all isomeric alcohols of molecular formula, C5H12O are shown below:
Structural isomers:- Compounds that have different structural formula but the same molecular formula and different IUPAC name are called structural isomers
C5H120 represents eight isomeric alcohols
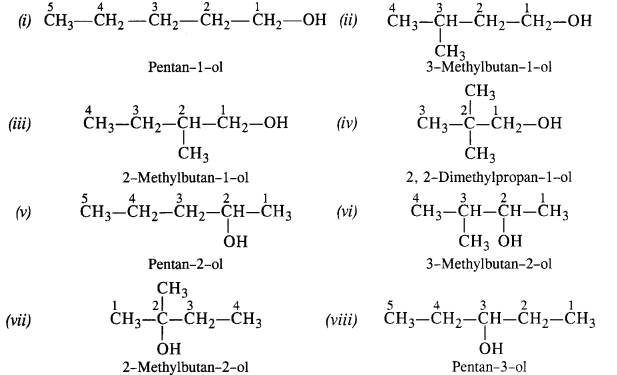
(ii) Primary alcohol: −OH group attached with 1 o -carbon Pentan–1–ol;
2–Methylbutan–1–ol; 3–Methylbutan–1–ol; 2,2– Dimethylpropan–1–ol
Secondary alcohol: −OH group attached with 2 o -carbon
Pentan–2–ol; 3–Methylbutan-2–ol; Pentan–3–ol
Tertiary alcohol: −OH group attached
with 3 o -carbon 2–methylbutan–2–ol
11.4 Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Ans – Although the molecular masses of propanol (Propan-l-ol) and butane are similar, 60m and 58u, respectively, propanol has a higher boiling point (391 K) due to intermolecular hydrogen bonding. However, because butane lacks a polar OH group, it is not present. Weak van der Waals forces are the sole attracting forces. As a result, propanol has a higher boiling point than butane (309 K)
11.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Ans – Two things can be said to explain why alcohols dissolve in water:
(i) They both have polar properties.
(ii) Intermolecular hydrogen bonds are formed between both of their molecules.
Hydrocarbons do not form hydrogen bonds with alcohols because they are non-polar and non-reactive. Hydrocarbons are almost insoluble in water, whereas alcohols easily dissolve in it.

11.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Ans – The hydroboration oxidation process is defined as the addition of borane and subsequent oxidation.
Example: When the chemical propene undergoes a hydroboration-oxidation process, propan-1-ol is the product. In this reaction, trialkyl borane is produced as an electrophilic addition product when propene interacts with diborane (BH3)2. Immediately after this electrophilic addition comes the anti-Markownikoff rule. In the presence of aqueous sodium hydroxide, hydrogen peroxide converts this electrophilic addition product to alcohol.
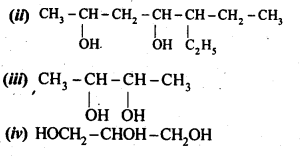
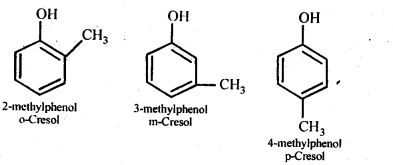
11.7 Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Ans –
11.8 While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Ans – While p-nitrophenol is not steam volatile, o-nitrophenol is. This is due to intramolecular hydrogen bonding in the o-nitrophenol molecules. As a result, it has a lower boiling point than p-nitrophenol, where intermolecular hydrogen bonds hold the molecules together.
It’s noteworthy to notice that the kind and position of the substituent in substituted phenols affect the boiling point of phenol.
For illustration.
While p-nitrophenol is not steam volatile, o-nitrophenol is. This is corroborated by the fact that o-nitrophenol has a lower boiling point temperature (100°C) than p-nitrophenol (279°C). In the chemical compound o-nitrophenol, the neighbouring OH and NO2 groups form intramolecular hydrogen bonds. In the p-isomers, these are nevertheless connected by intermolecular hydrogen bonding.

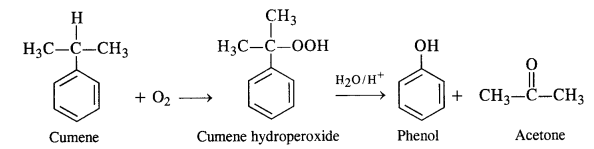
11.9 Give the equations of reactions for the preparation of phenol from cumene.
Ans – To make phenol, cumene must first undergo an oxidation reaction with cumene hydro-peroxide in the presence of air.
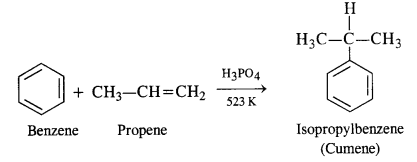
Then, cumene hydroxide is treated with weak acid to produce the by-products phenol and acetone.
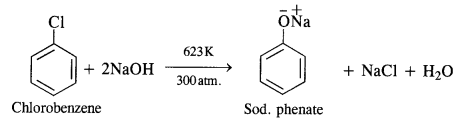
11.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
Ans – Under the conditions of 623 K temperature and 320 atm pressure, chlorobenzene is fused with sodium hydroxide to produce sodium phenoxide, which upon acidification yields phenol.
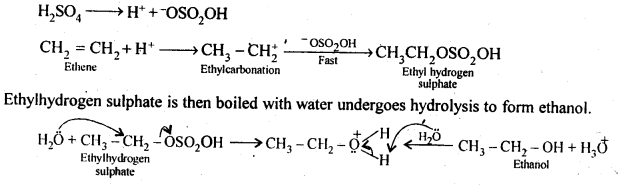
11.11 Write the mechanism of hydration of ethene to yield ethanol.
Ans – In the presence of an acid, there is no direct addition of water to ethene. Ethyl hydrogen sulphate is created indirectly by passing concentrated H2S04 via ethene first.
11.12 You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Ans – The reaction of Benzene with concentrated sulphuric acid is called sulphonation.

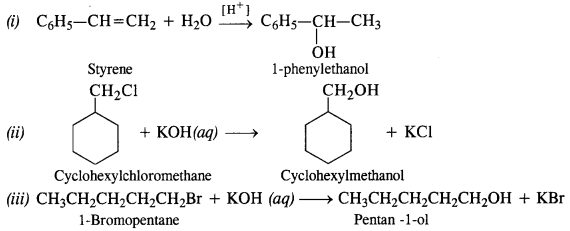
11.13 Show how will you synthesise:
(i) 1-phenylethanol from a suitable alkene.
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) pentan-1-ol using a suitable alkyl halide?
Ans –
11.14 Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Ans – The acidity of phenols. Weakly acidic by nature, phenols. Carbolic acid is the liquid version of phenol that contains around 5% water. At 298 K, phenol’s dissociation constant (Ka) is 10-10. (room temperature). 10.0 is the matching pKa* value. The following characteristics reveal an acidic nature:
(a) Reaction with sodium:
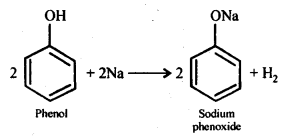
(b) Reaction with NaOH:
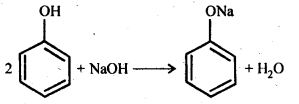
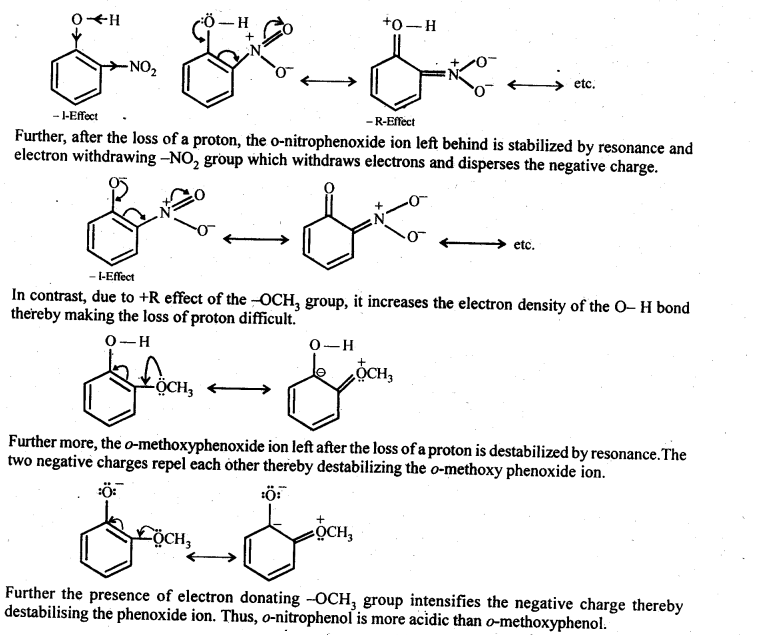
11.15 Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
Ans – An electron-withdrawing group is the nitro group (-M group). The electron density in the O–H bond is reduced by the presence of nitro group in the ortho position. As a result, proton loss is simpler. Additionally, resonance stabilises the o-nitrophenoxide ion that results from the loss of protons. As a result, ortho nitrophenol is a more potent acid. so the resulting anion is more stable. Thus, the character’s acidity (+M) rises.
11.16 Explain how does the –OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Ans –

The +R action of the -OH group raises the electron density in the benzene ring, which makes it easier for an electrophile to attack. In other words, the benzene ring is activated for electrophilic substitution processes by the presence of the -OH group. Additionally, electrophilic substitution happens mostly at the o- and p-positions because of the relative higher electron density at the two o- and one p-position.
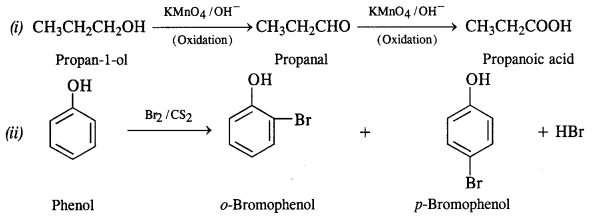
11.17 Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline KMnO4 solution.
(ii) Bromine in CS2 with phenol.
(iii) Dilute HNO3 with phenol.
(iv) Treating phenol wih chloroform in presence of aqueous NaOH.
Ans – (i) Oxidation of propan-1-ol with alkaline KMnO4 solution gives propanoic acid
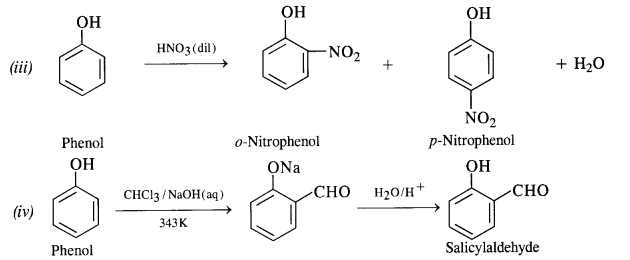
11.18 Explain the following with an example.
(i) Kolbe’s reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
Ans – (i) Kolbe reaction.
Sodium phenoxide is created when sodium hydroxide is used to treat phenol. When this sodium phenoxide is combined with carbon dioxide and subjected to acidification and electrophilic substitution, the major result is ortho-hydroxybenzoic acid. Kolbe’s reaction is the name given to this process.
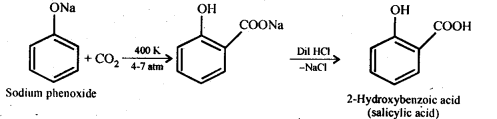
(ii) Reimer-Tiemann reaction.
A CHO group is added to the ortho position of the benzene ring when chloroform (CHCl3) is used to treat phenol in the presence of sodium hydroxide.
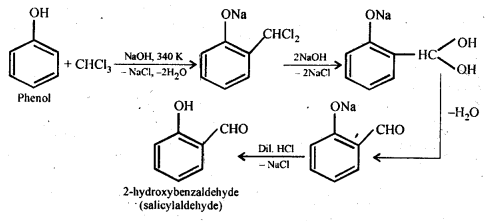
(iii) Williamson ether synthesis.
Alkyl halides can combine with sodium alkoxides to produce symmetrical and asymmetrical ethers using the Williamson ether synthesis technique.
(iv) Unsymmetrical ethers: These ethers differ in that they include an unequal number of carbon alkyl atoms in each of the two alkyl groups on either side of an oxygen atom. Take ethyl methyl ether, for instance (CH3 O CH2 CH3).
11.19 Write the mechanism of acid dehydration of ethanol to yield ethene.
Ans – The relative stabilities of the carbocations created in the slow stage can also be used to support the relative ease of dehydration of various alcohols, including primary, secondary, and tertiary alcohols. The tertiary alcohols are the most reactive by nature, whereas the primary alcohols are the least reactive, because the tertiary carbocation is the most stable and the primary is the least.

11.20 How are the following conversions carried out?
(i) Propene → Propan-2-ol.
(ii) Benzyl chloride → Benzyl alcohol.
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
Ans –
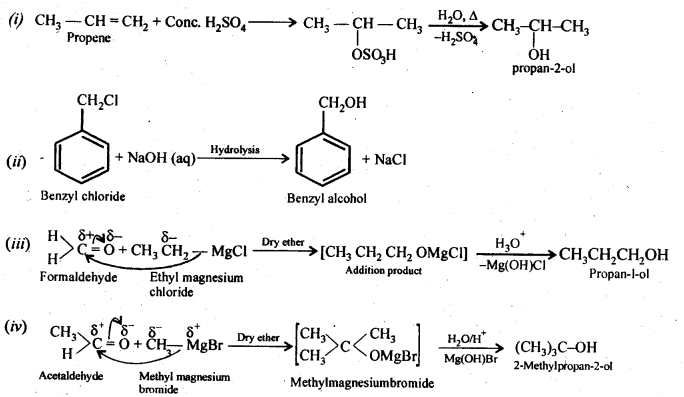
11.21 Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Bromination of phenol to 2,4,6-tribromophenol.
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-ol to propene.
(vi) Butan-2-one to butan-2-ol.
Ans – (i) Acidified potassium dichromate or neutral/ acidic/ alkaline potassium permanganate.
John’s reagent (CrO3 H + ⁄ or CrO3⁄H2SO4 ),HNO3
(ii) Pyridinium chlorochromate (PCC), (C5H5NH)+ ClCrO3– in CH2Cl2
or Pyridinium dichromate (PDC),[(C5H5NH)2]2+Cr2O72-in CH2Cl2
Mild oxidizing agent like pyridium chloro chromate (PCC),
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate.
(v) 85% H2S04 at 440 K.
(vi) Ni/H2 or NaBH4 or LiAlH4.
Butan-2-one to butan-2-ol
11.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Ans – The boiling point is dependent on the attraction force.
Due to the OH group’s presence in ethanol, intermolecular H-bonding occurs, which causes molecules to associate. These hydrogen bonds need to be broken with additional energy. Methoxymethane, on the other hand, does not experience H-bonding. Vanderwall force of attraction holds it together (dipole -dipole attraction). The force of a vander wall is weaker than an H-bond. As a result, ethanol has a greater boiling point than methoxymethane.
11.23 Give IUPAC names of the following ethers:
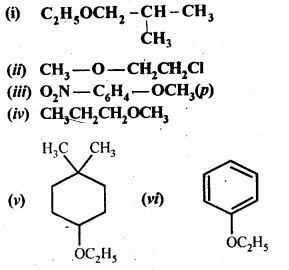
Ans – (i) 1-Methoxy-2-methylpropane
(ii) 1-Chloro-2-methoxy ethane
(iii) 4-Nitroanisole
(iv) 1-Methoxypropane
(v) 4-Ethoxy-1, 1-dimethyl cyclohexane
(vi) Ethoxybenzene
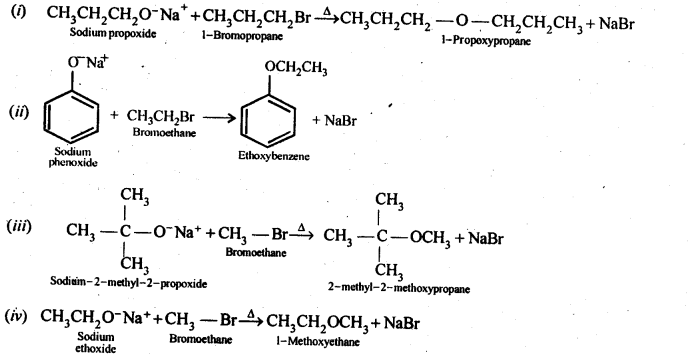
11.24 Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(i) 1-Propoxypropane
(ii) Ethoxybenzene
(iii) 2-Methoxy-2-methylpropane
(iv) 1-Methoxyethane
Ans –
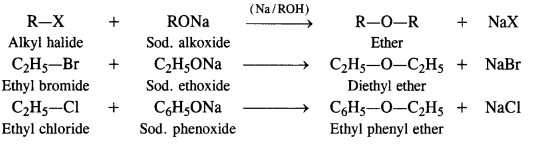
11.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Ans – The versatile Williamson synthesis can be used to create both symmetrical and unsymmetrical ethers. However, a good selection of reactants is required for the synthesis of unsymmetrical ethers. The best yields of unsymmetrical ethers are obtained when the alkyl halides are primary, while the alkoxide may be primary, secondary, or tertiary. This is because Williamson’s synthesis proceeds by SN2 mechanism and primary alkyl halides are most reactive in Sn2 reaction. For instance, ethyl bromide is treated with sodium tert-butoxide to create tert-butylethyl ether.
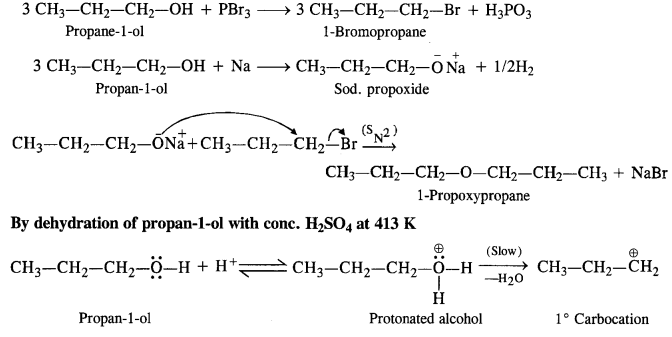
11.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Ans – 1-propoxypropane can be synthesized from propan-1-ol by dehydration. Propan-1-ol undergoes dehydration in the presence of protic acids (such as H2SO4,H3PO4) to give 1-propoxypropane.
11.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Ans – Alcohol molecules attack alcohol molecules that have undergone protonation in a bimolecular process (SN2) that results in the creation of ethers. The alkyl group should not be obstructed during the procedure. The alkyl group is inhibited in secondary or tertiary alcohols. As a result, substitution is outweighed by elimination. As a result, alkenes rather than ethers are produced.
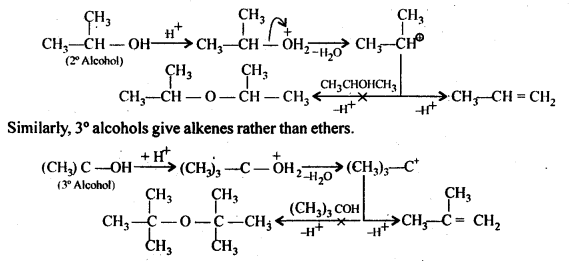
However, under these circumstances, 2° and 3° alcohols yield alkenes as opposed to ethers. The reason for this is that the alcohol molecule’s nucleophilic attack on the protonated alcohol molecule is prevented by steric hindrance. To create stable 2° and 3° carbocation, protonated 2° and 3° alcohols instead lose a water molecule.
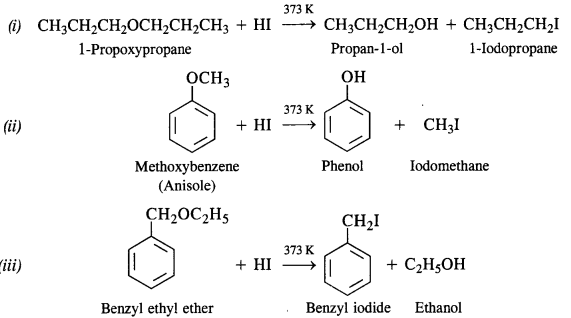
11.28 Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane
(ii) methoxybenzene and
(iii) benzyl ethyl ether.
Ans –
11.29 Explain the fact that in aryl alkyl ethers
(i) the alkoxy group activates the benzene ring towards electrophilic substitution and
(ii) it directs the incoming substituents to ortho and para positions in benzene ring.
Ans – (i) The ortho and para locations in the ring are activated by the alkoxy group’s (RO -) lone electron pair on the oxygen atom through the + M (or + R) effect, as shown below:
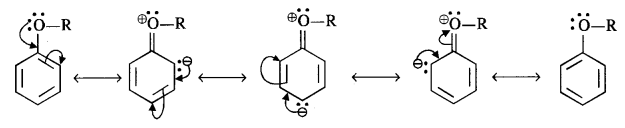
(ii) The alkoxy group guides the electrophile entering group toward the orthogonal and paragonal places in the ring. A combination of isomeric products is produced as a result.
11.30 Write the mechanism of the reaction of HI with methoxymethane.
Ans – The mechanism of the reaction of HI with methoxymethane is SN2

11.31 Write equations of the following reactions:
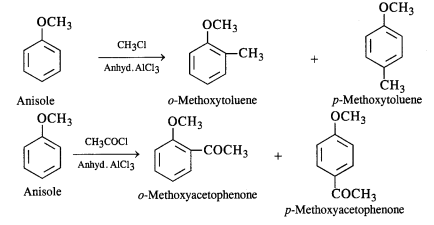
(i) Friedel-Crafts reaction – alkylation of anisole.
(ii) Nitration of anisole.
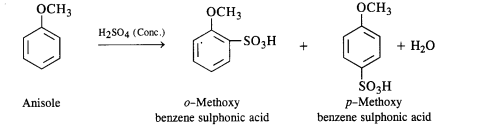
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft’s acetylation of anisole.
Ans –
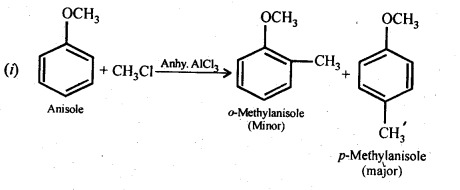
−OCH3 group is electron releasing (+M) group. So, it increases electron density at ortho and para. So, electrophilic R + (Carbonation) attack at ortho and para. Para will be major than ortho due to less hindrance
(ii) The nitration of anisole carried with a nitrating mixture of conc. UNO3 and conc. H2SO4 upon heating gives a
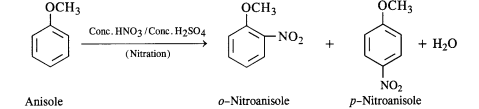
(iii)
(iv)
11.32 Show how would you synthesise the following alcohols from appropriate alkenes?
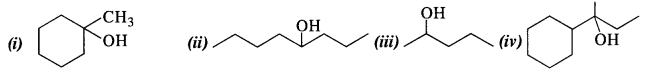
Ans – Alkenes are hydrated in an acidic media to create all alcohols. Following Markownikov’s rule, the addition. The reaction allows for the utilisation of 1-methylcyclohexene.
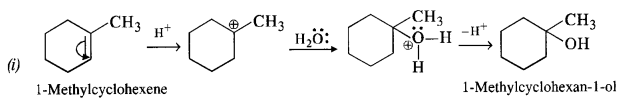
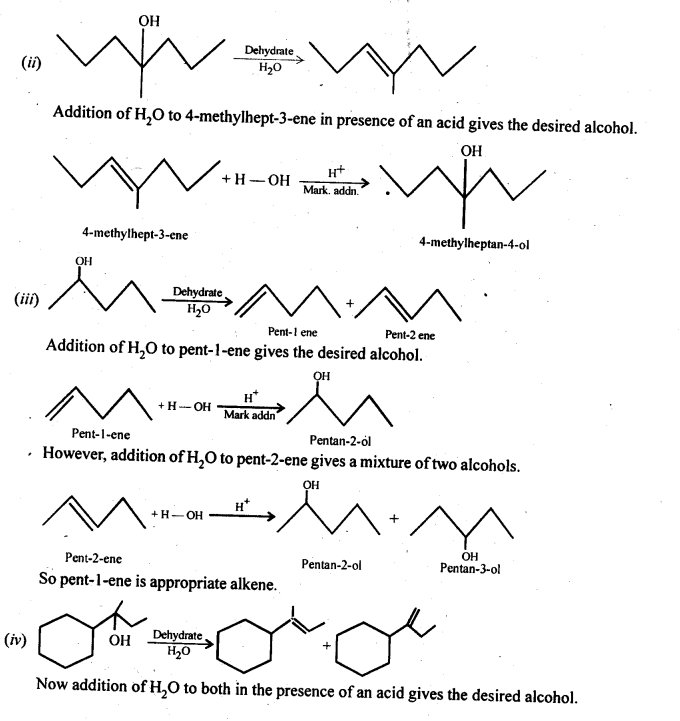
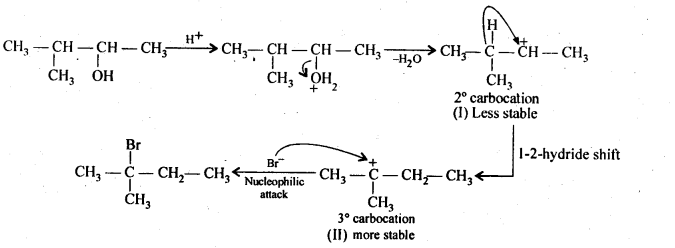
11.33 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place:
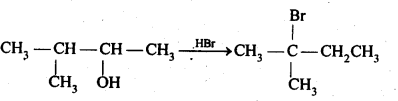
Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
Ans –