NCERT Exercise Solutions – Chemistry Chapter 1 The Solid State
1.1 Define the term ‘amorphous’. Give a few examples of amorphous solids.
Ans- Amorphous solids have the constituent particles arranged only in short-range order and consequently, they behave like supercooled liquids. So do not have sharp melting points. Also, they are isotropic in nature i.e., their physical properties are the same in all directions.
E.g. quartz glass, polymers, gels etc.
1.2 What makes a glass different from a solid such as quartz? Under what conditions could quartz be converted into glass?
Ans- The arrangement of the constituent particles makes glass different from quartz. In glass, the constituent particles have short range order, but in quartz, the constituent particles have both long range and short range orders. Quartz can be converted into glass by heating and then cooling it rapidly.
1.3 Classify each of the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
(i) Tetra phosphorus decoxide (P4O10)
(ii) Ammonium phosphate (NH4 ) 3 PO4
(iii) SiC (ix) Rb
(iv) I 2 (x) LiBr
(v) P4 (xi) Si
(vi) Plastic
(vii) Graphite
(viii) Brass
Ans- Ionic → (ii) Ammonium phosphate (NH4)3PO4, (x) LiBr
Metallic → (viii) Brass, (ix) Rb
Molecular → (i) Tetra phosphorus decoxide (P4O10), (iv) I2, (v) P4.
Covalent (network) → (iii) SiC, (vii) Graphite, (xi) Si
Amorphous → (vi) Plastic
1.4 (i) What is meant by the term ‘coordination number’?
(ii) What is the coordination number of atoms:
(a) in a cubic close-packed structure?
(b) in a body-centered cubic structure?
Ans- (i) It is defined as the number of nearest neighbors of particle in a close packed structure.
(ii) (a) In a cubic close packed structure the coordination number is 12.
(b) In a body centered cubic close structure the coordination number is 8.
1.5 How can you determine the atomic mass of an unknown metal if you know its density and the dimension of its unit cell? Explain.
Ans- Let us assume a cubic crystal, then Its volume of unit cell will be = a3 &
No of atom in each cell = z
Also molar mass of the metal =M,
Mass of each atom = m
Now we know, mass of unit cell = no of atom in each cell x Mass of each atom ——————-(1)
Also Mass of each atom = molar mass / avagadro number ,i.e M/NA ————(2)
Putting the values of 2 in 1 we get,
Mass of unit cell = Mz / NA ——————(3)
Now density of unit cell (d) = mass of unit cell / volume of unit cell
Therefore from 1,2,3 we,get
d = Mz / a3 NA—————————(4)
From 4th equation we can calculate the atomic mass of unknown number.
1.6 ‘Stability of a crystal is reflected in the magnitude of its melting points’. Comment. Collect melting points of solid water, ethyl alcohol, diethyl ether and methane from a data book. What can you say about the intermolecular forces between these molecules?
Ans- Stability of a crystal is reflected in the magnitude of its melting points because higher the melting point, greater is the intermolecular force and greater the intermolecular force greater is the stability. And hence, a substance with higher melting point would be more stable.
The melting points of the given substances are as follows:
Solid water – 273 K
Ethyl alcohol – 158.8 K
Diethyl ether – 156.85 K
Methane – 89.34 K
As we can see the melting point of solid water is highest and melting point of methane is lowest among the given substance. This says that intermolecular force in solid water is strongest and the intermolecular force in methane is weakest.
1.7 How will you distinguish between the following pairs of terms:
(i) Hexagonal close-packing and cubic close-packing?
(ii) Crystal lattice and unit cell?
(iii) Tetrahedral void and octahedral void?
Ans- (i)
| Hexagonal close packing | Cubic close packing |
|---|---|
| It is 2dimensional packing | It is a 3 dimensional packing |
| Here the sphere of 2nd row sit into the depressions in between the spheres of the 1st row | Here the sphere in the third layer is placed over hollows voids of the 1st layer |
| It is abbrebiated as hcp | It is abbrebiated as ccp |
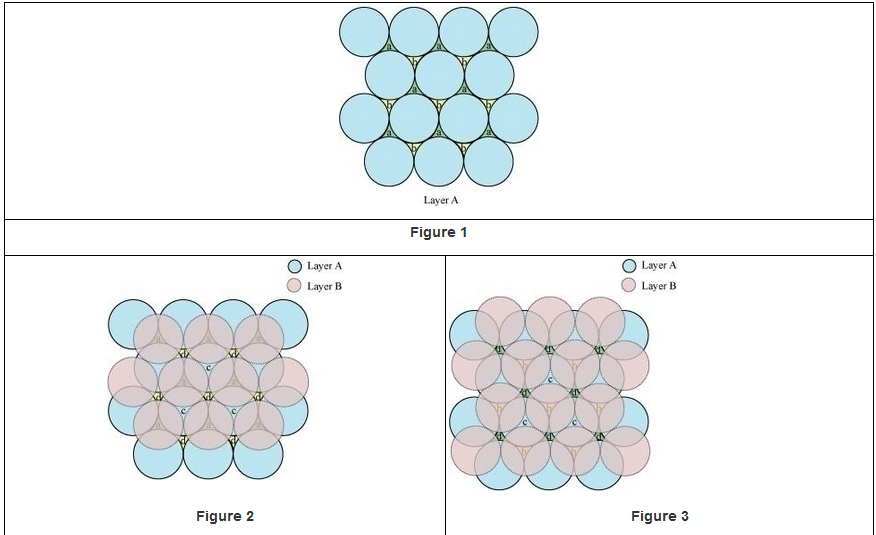
A 2-D hexagonal close-packing contains two types of triangular voids (a and b) as shown in figure 1. Let us call this 2-D structure as layer A. Now, particles are kept in the voids present in layer A (it can be easily observed from figures 2 and 3 that only one of the voids will be occupied in the process, i.e., either a or b). Let us call the particles or spheres present in the voids of layer A as layer B. Now, two types of voids are present in layer B (c and d).
(ii)
| Crystal lattice | Unit cell |
|---|---|
| It a regular 3 dimensional arrangement of the points in a crystal | It is smallest but complete unit in the space lattice which is when repeated over & again in 3 dimensions |
(iii)
| Tetrahedral void | Octahedral void |
|---|---|
| It is a vacant space among four spheres having tetrahedral arrangement | It is avoid formed by 2 equilateral triangles with apices in opposite directions |
| There are 2 tetrahedral sites for each sphere | There is only 1 octahedral |
1.8 How many lattice points are there in one unit cell of each of the following lattice?
(i) Face-centered cubic
(ii) Face-centered tetragonal
(iii) Body-centered
Ans- (i) There are 14 (8 from the corners + 6 from the faces) lattice points in face-centred cubic.
(ii) There are 14 (8 from the corners + 6 from the faces) lattice points in face-centred tetragonal.
(iii) There are 9 (1 from the centre + 8 from the corners) lattice points in body-centred cubic.
1.9 Explain
(i) The basis of similarities and differences between metallic and ionic crystals.
(ii) Ionic solids are hard and brittle.
Ans- (i) The basis of similarities between metallic and ionic crystals is that both these crystal types are held by the electrostatic force of attraction. In metallic crystals, the electrostatic force acts between the positive ions and the electrons. In ionic crystals, it acts between the oppositely-charged ions. Hence, both have high melting points. The basis of differences between metallic and ionic crystals is that in metallic crystals, the electrons are free to move and so, metallic crystals can conduct electricity. However, in ionic crystals, the ions are not free to move. As a result, they cannot conduct electricity. However, in molten state or in aqueous solution, they do conduct electricity.
(ii) The constituent particles of ionic crystals are ions. These ions are held together in three-dimensional arrangements by the electrostatic force of attraction. Since the electrostatic force of attraction is very strong, the charged ions are held in fixed positions. This is the reason why ionic crystals are hard and brittle.
1.10 Calculate the efficiency of packing in case of a metal crystal for
(i) simple cubic
(ii) body-centered cubic
(iii) face-centered cubic (with the assumptions that atoms are touching each other).
Ans- (i) Simple cubic:
Suppose the edge length of the unit cell = a
&
Radius of the sphere = r
Then,since the sphere are touching each other along the edge,therefore a = 2r
Now there are 8 spheres at the corners of the cube & each sphere at the corner is shared by 8 unit cells & the contribution per unit cell is 1/8 so that
Number of spheres per unit cell is 8 x 1/8 = 1
Volume of sphere =4/3πr3 & volume of cube = a3 = (2r)3 = 8r3
Now packing efficiency = (volume of one sphere / total volume of cubic unit cell) x 100
Or
(4/3 πr3 / 8r3) x 100 = 52.4%
Therefore the volume occupied in simple cubic arrangement = 52.4%
(ii) Body centered cubic:
Let us suppose the edge leght = a & radius of each sphere = r then there are 8 spheres at the corners & 1 in the body of unit cell
Therefore number of spheres per unit cell = (8 x1/8) + 1 = 2
Now volume of unit cell = a3 = (4r / √3)3
and volume of a sphere = 4 / 3πr3
Total volume of two spheres = 2 x 4/3πr3
Packing efficiency = (volume of two spheres in unit cell/total volume of unit cell ) x 100
= (2 x 4/3πr3 / (4r/√3)3 ) x 100 = 68%
Therefore volume occupied in bcc arrangement = 68%
(iii) Face centered:
let us suppose the edge length of the unit cell = a
Radius of each sphere = r
Now there are 8 spheres at the corner & 6 at the faces
Therefore number of spheres in unit cell = (8 x 1/8 + 6 x1/2) = 4
From the arrangement of fcc, we get a = 2√2r
Now volume of a unit cell = a3 = (2√2r)3 = 16√2r3
Total volume of 4 spheres = 4 x 4/3 πr3 = 16/3 πr3
Packing efficiency = (volume of 4 spheres in the unit cell/total volume of unit cell) x 100
= (16/3 πr3 /16√2r3) x 100 = 74%
Therefore volume occupied in fcc = 74%
1.11 Silver crystallises in fcc lattice. If edge length of the cell is 4.07 × 10–8 cm and density is 10.5 g cm–3, calculate the atomic mass of silver.
Ans- Given, edge length (a) =4.07×10−8=4.07×10-8
Density (d) = 10.5 g cm-3
For fcc number of atoms per unit cell (z) = 4
We know that Atomic Mass (M) =da3NAz=da3NAz
Thus, M = 10.5gcm3×4.07×10−8×6.033×1023mol−1410.5gcm3×4.07×10-8×6.033×1023mol-14
Or, M = 10.5g×67.419×10−24×6.022×1023mol−1410.5g×67.419×10-24×6.022×1023mol-14
Or, M = 10.5g×67.419×10−1×6.022mol−1410.5g×67.419×10-1×6.022mol-14
Or, M =426.29707894gmol−1=426.29707894gmol-1
Or, M=106.574gmol−1=107gmol−1
1.12 A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body-center. What is the formula of the compound? What are the coordination numbers of P and Q?
Ans-
It is given that the atoms of Q are present at the corners of the cube.
Therefore, number of atoms of Q in one unit cell = 8 x (1/8) = 1
It is also given that the atoms of P are present at the body-centre.
Therefore, number of atoms of P in one unit cell = 1
This means that the ratio of the number of P atoms to the number of Q atoms,
P:Q = 1:1
Hence, the formula of the compound is PQ.
The coordination number of both P and Q is 8.
1.13 Niobium crystallises in body-centred cubic structure. If density is 8.55 g cm–3, calculate atomic radius of niobium using its atomic mass 93 u.
Ans-
Given Density = 8.55 gcm-3
Let the length of the edge = a cm
Number of atoms per unit cell, Z = 2
Atomic mass M = 93 u
From the formula , density = ZxM/a3 x N0, we get
8.55 = 2 x 93/a3 x 6.022 x 1023 = 36.12 x 10-24 cm3
Now edge length,a = (36.12 x 10-24)1/3 = 3.306 x 10-8 cm
Now radius in body centered cubic, r = √3/4 a
Putting the value of a, we get .r = 1.431 x 10-10 m
1.14 If the radius of the octahedral void is r and radius of the atoms in closepacking is R, derive relation between r and R.
Ans-
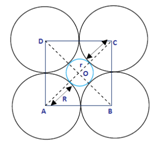
Now by Pythagoras theorem, we have
2R2 = (R+ r)2 + (R+ r)2
Or
4R2 = 2(R +r)2
TAKING ROOT BOTH SIDES ,WE GET
R√2 = R +r
Or
R√2 – R = r
Or
r = R√2 – R
Putting the value of √2, we get .
r = R(1.414-1)
r = 0.414R
1.15 Copper crystallises into a fcc lattice with edge length 3.61 × 10–8 cm. Show that the calculated density is in agreement with its measured value of 8.92 g cm–3.
Ans: Relation between density and edge length gives:
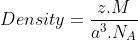
Since it crystallises in fcc lattice, thus z = 4.
Molar mass of copper = 63.546 u.
So,
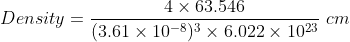
1.16 Analysis shows that nickel oxide has the formula Ni0.98O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?
Ans- The formula of nickel oxide is Ni0.98O1.00.
Therefore, the ratio of the number of Ni atoms to the number of O atoms,
Ni : O = 0.98 : 1.00 = 98 : 100
Now, total charge on 100 O2− ions = 100 × (−2)
= −200
Let the number of Ni2+ ions be x.
So, the number of NI3+ ions is 98 − x.
Now, total charge on Ni2+ ions = x(+2)
= +2x
And, total charge on NI3+ ions = (98 − x)(+3)
= 294 − 3x
Since, the compound is neutral, we can write:
2x + (294 − 3x) + (−200) = 0
⇒ −x + 94 = 0
⇒ x = 94
Therefore, number of Ni2+ ions = 94
And, number of NI3+ ions = 98 − 94 = 4
Hence, fraction of nickel that exists as Ni2+ = 94/98
= 0.959
And, fraction of nickel that exists as = NI3+ = 4/98
= 0.041
Alternatively, fraction of nickel that exists as NI3+ = 1 − 0.959
= 0.041
1.17 What is a semiconductor? Describe the two main types of semiconductors and contrast their conduction mechanism.
Ans- Semiconductors are substances having conductance in the intermediate range of 10^-6 to 10^4 ohm^−1m^−1. The two main types of semiconductors are: (i) n-type semiconductor (ii) p-type semiconductor
(i)n-type semiconductor: The semiconductor whose increased conductivity is a result of negatively charged electrons is called an n-type semiconductor. When the crystal of a group 14 element such as Si or Ge is doped with a group 15 element such as P or As, an n-type semiconductor is generated. Si and Ge have four valence electrons each. In their crystals, each atom forms four covalent bonds. On the other hand, P and As contain five valence electrons each. When Si or Ge is doped with P or As, the latter occupies some of the lattice sites in the crystal. Four out of five electrons are used in the formation of four covalent bonds with four neighboring Si or Ge atoms. The remaining fifth electron becomes delocalized and increases the conductivity of the doped Si or Ge.
(ii)p-type semiconductor: The semiconductor whose increased in conductivity is a result of electron hole is called a p-type semiconductor. When a crystal of group 14 elements such as Si or Ge is doped with a group 13 element such as B, Al, or Ga (which contains only three valence electrons), a p-type of semiconductor is generated. When a crystal of Si is doped with B, the three electrons of B are used in the formation of three covalent bonds and an electron hole is created. An electron from the neighboring atom can come and fill this electron hole, but in doing so, it would leave an electron hole at its original position. The process appears as if the electron hole has moved in the direction opposite to that of the electron that filled it. Therefore, when an electric field is applied, electrons will move toward the positively-charged plate through electron holes. However, it will appear as if the electron holes are positively-charged.
1.18 Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1. Can you account for the fact that this substance is a p-type semiconductor?
Ans- From the given data it is clear that the ratio of copper to oxygen is less than 2:1. Therefore, cuprous oxide is non stoichiometric crystal. This means that some Cu+ ions have been replaced by Cu2+ ions and to maintain electrical neutrality, every 2 Cu+ ions will be replaced by one Cu2+ ions, making a hole. Since the conduction will be due to the presence of these positive holes, the given substance is a p-type semi- conductor
1.19 Ferric oxide crystallises in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Ans- Let the number of oxide (O2−) ions be x.
So, number of octahedral voids = x
It is given that two out of every three octahedral holes are occupied by ferric ions.
So, number of ferric (Fe3+) ions = (2/3)x
Therefore, ratio of the number of Fe3+ ions to the number of O2− ions,
Fe3+ : O2− = (2/3)x:x = (2/3):1 = 2 : 3
Hence, the formula of the ferric oxide is Fe2O3.
1.20 Classify each of the following as being either a p-type or a n-type semiconductor:
(i) Ge doped with In (ii) Si doped with B.
Ans- (i) Ge (a group 14 element) is doped with In (a group 13 element). Therefore, a hole will be created and the semiconductor generated will be a p-type semiconductor.
(ii) B (a group 13 element) is doped with Si (a group 14 element). So, there will be an extra electron and the semiconductor generated will be an n-type semiconductor.
1.21 Gold (atomic radius = 0.144 nm) crystallises in a face-centred unit cell. What is the length of a side of the cell?
Ans- Given, atomic radius = 0.144 nm
Type of unit cell = face centered
We know that side a in fcc =2√2r=22r
Thus, a=2√2×0.144nma=22×0.144nm
Or, a=2×1.414×0.144=407a=2×1.414×0.144=407 nm
Thus side of the given cell = 407 nm
1.22 In terms of band theory, what is the difference
(i) between a conductor and an insulator
(ii) between a conductor and a semiconductor?
Ans- (i) The valence band of a conductor is partially-filled or it overlaps with a higher energy, unoccupied conduction band. On the other hand, in the case of an insulator, the valence band is fully- filled and there is a large gap between the valence band and the conduction band
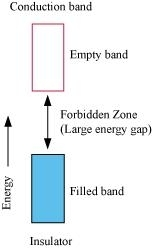
(ii) In the case of a conductor, the valence band is partially-filled or it overlaps with a higher energy, unoccupied conduction band. So, the electrons can flow easily under an applied electric field. On the other hand, the valence band of a semiconductor is filled and there is a small gap between the valence band and the next higher conduction band. Therefore, some electrons can jump from the valence band to the conduction band and conduct electricity.
1.23 Explain the following terms with suitable examples:
(i) Schottky defect (ii) Frenkel defect (iii) Interstitials and (iv) F-centres.
Ans- (i) Schottky defect: Schottky defect is basically a vacancy defect shown by ionic solids. In this defect, an equal number of cations and anions are missing to maintain electrical neutrality. It decreases the density of a substance. Significant number of Schottky defects is present in ionic solids. For example, in NaCl, there are approximately 106 Schottky pairs per cm3 at room temperature. Ionic substances containing similarsized cations and anions show this type of defect.
(ii) Frenkel defect: Ionic solids containing large differences in the sizes of ions show this type of defect. When the smaller ion (usually cation) is dislocated from its normal site to an interstitial site, Frenkel defect is created. It creates a vacancy defect as well as an interstitial defect. Frenkel defect is also known as dislocation defect. Ionic solids such as AgCl, AgBr, AgI, and ZnS show this type of defect.
(iii) Interstitials: Interstitial defect is shown by non-ionic solids. This type of defect is created when some constituent particles (atoms or molecules) occupy an interstitial site of the crystal. The density of a substance increases because of this defect.
(iv) F-centres: When the anionic sites of a crystal are occupied by unpaired electrons, the ionic sites are called F-centres. These unpaired electrons impart colour to the crystals. For example, when crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms are deposited on the surface of the crystal. The Cl ions diffuse from the crystal to its surface and combine with Na atoms, forming NaCl. During this process, the Na atoms on the surface of the crystal lose electrons.
1.24 Aluminum crystallises in a cubic close-packed structure. Its metallic radius is 125 pm.
(i) What is the length of the side of the unit cell?
(ii) How many unit cells are there in 1.00 cm3 of aluminum?
Ans- In a cubic close packed structure,the length of the side of unit cell is related to radius by an equation r = a/2√2
Or
a = r x 2√2
Putting the values in the equation, we get
a = 125 x 2 x 1.414 = 353.5 pm
Volume of the unit cell= (353.5 x 10-10)3 = 4.42 x 10-23
Number of unit cells in 1 cm3 = 1 / 4.42 x 10-23 = 2.26 x 1022 unit cells
1.25 If NaCl is doped with 10–3 mol % of SrCl2 , what is the concentration of cation vacancies?
Ans- Given that NaCl is doped with 10−3 mol% of SrCl2.
This means that 100 mol of NaCl is doped with 10−3 mol of SrCl2.
Therefore, 1 mol of NaCl is doped withmol of SrCl2
= 10−5 mol of SrCl2
Cation vacancies produced by one Sr2+ ion = 1

1.26 Explain the following with suitable examples:
(i) Ferromagnetism (ii) Paramagnetism (iii) Ferrimagnetism (iv) Antiferromagnetism (v) 12-16 and 13-15 group compounds.
Ans- (i) Ferromagnetism – Substances that are attracted strongly with magnetic field are called ferromagnetic substances, such as cobalt, nickel, iron, gadolinium, chromium oxide, etc. Ferromagnetic substances can be permanently magnetized also.
Metal ions of ferromagnetic substances are randomly oriented in normal condition and substances do not act as a magnet. But when metal ions are grouped together in small regions, called domains, each domains act like a tiny magnet and produce strong magnetic field, in such condition ferromagnetic substance act like a magnet. When the ordering of domains in group persists even after removal of magnetic field a ferromagnetic substance becomes a permanent magnet.
(ii) Paramagnetism – Substances which are attracted slightly by magnetic field and do not retain the magnetic property after removal of magnetic field are called paramagnetic substances. For example O2, Cu2+, Fe3+, Cr3+, Magnesium, molybdenum, lithium, etc.
Substances show paramagnetism because of presence of unpaired electrons. These unpaired electrons are attracted by magnetic field.
(iii) Ferrimagnetism – Substances which are slightly attracted in magnetic field and in which domains are grouped in parallel and anti-parallel direction but in unequal number, are called ferromagnetic substances and this property is called ferrimagnetism.
For example, magnetite (Fe3O4), ferrite (MgFe2O4), ZnFe2O4, etc.
Ferrimagnetic substances lose ferrimagnetism on heating and become paramagnetic.
(iv) Antiferromagnetism – Substances in which domain structure are similar to ferromagnetic substances but are oriented oppositely, which cancel the magnetic property are called antiferromagnetic substances and this property is called antiferromagnetism. For example; MnO.
(v) 12-16 and 13-15 group compounds – Compounds belong to 12 – 16 group are formed by the combination of elements of 12 and 16 groups. For example – ZnS, Cds, etc.
Compounds belong to 13 – 15 group are formed by the combination of the elements of 13 and 15 groups. For example – InSb, GaAs, etc.
Bonds in these compounds are not perfectly covalent.





