NCERT Exercise Solutions – Chemistry Chapter 14 Biomolecules
14.1 What are monosaccharides?
Ans – The simplest form of a carbohydrate is a monosaccharide. They are the fundamental units of more complex carbohydrates and are composed of hydrogen, carbon, and oxygen atoms.
Aldoses are monosaccharides with an aldehyde group, while those with a Ketones are a subgroup of the keto diet. Trioses are another classification for monosaccharides. tetroses, pentoses, hexoses, and heptoses based on how many carbon atoms they contain . For instance, an aldose and a ketose with three carbon atoms each have the name ketotriose. Aldotriose is a compound that has three carbon atoms.
To create and store energy, monosaccharides are utilized. most living things generate energy through reducing the monosaccharide glucose and obtaining the energy produced by Bonds. Monosaccharides include sugars including galactose, fructose, and glucose.
14.2 What are reducing sugars?
Ans – Sugars that can reduce substances are known as reducing sugars. They have a reducing group inside of them, which could be either an aldehydic (-CHO) or a ketonic (>C=0) group. Tollen’s reagent and Fehling solution are the two common ways that reducing sugars react. These reactions are not shown by non-reducing carbohydrates. For instance, lowering sugars include glucose, fructose, lactose, etc. Because glucose and fructose are linked by their aldehydic and ketonic groups by glycosidic linkage, sucrose is classified as a non-reducing sugar. Sucrose is a non-reducing sugar because these groups are not free to move around.
14.3 Write two main functions of carbohydrates in plants.
Ans – In plants, carbohydrates serve two basic purposes.
(i) Starch and other polysaccharides act as storage molecules.
(ii) The carbohydrate cellulose is employed to construct the cell wall.
14.4 Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Ans – Monosaccharides: Ribose, 2-deoxyribose, galactose, fructose.
Disaccharides: Maltose, lactose.
14.5 What do you understand by the term glycosidic linkage?
Ans – When a water molecule is lost, a link known as a glycosidic linkage is created between two monosaccharide units. This link is made up of an oxygen atom. It is the formation of a connection between two monosaccharides by the condensation of their hydroxyl groups.
Two monosaccharide units, glucose and fructose, are found in a sucrose molecule and are linked together by a glycosidic connection.
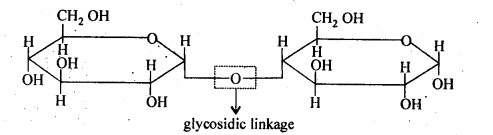
14.6 What is glycogen? How is it different from starch?
Ans – As a carbohydrate, glycogen (polysaccharide). Animals store carbs as glycogen. Animal bodies store the carbohydrates as glycogen. It is also known as animal starch, and because of the similarity between its structure and that of amylopectin, it is a branched chain polymer of -D-glucose units. The chain is produced by C1 – C4 glycosidic linkage, whereas branching is caused by the creation of C1 – C6 glycosidic linkage. The length of the chain is one of the primary distinctions between glycogen and amylopectin. In glycogen, there are only 10–14 molecules of D-glucose present, whereas the chain in amylopectin contains 20–25 molecules. Amylopectin is less branched than glycogen. It is mostly found in the liver, muscles, and brain.
Two different carbohydrates, amylose (15–20%) and amylopectin (80–85%), make up starch.
However, there is just one part of glycogen that resembles amylopectin in structure. Also, compared to amylopectin, glycogen has more branches.
14.7 What are the hydrolysis products of
(i) sucrose and
(ii) lactose?
Ans – When starch is hydrolyzed, it produces – D(+) glucose, a component of both amylose and amylopectin. Galactose and glucose are produced when lactose is hydrolyzed.
Only D(+) glucose results from the hydrolysis of cellulose. This indicates that cellulose contains exclusively D(+) glucose units, however unlike starch, these are -D(+) glucose molecules rather than a-D(+) glucose molecules. According to the results of the X-ray study, bundles of huge linear chains of 3 – D(+) glucose molecules are present and are held together by hydrogen bonds formed between nearby hydroxyl groups.
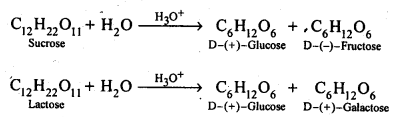
14.8 What is the basic structural difference between starch and cellulose?
Ans – Starch is not a single substance. Both amylose and amylopectin are also present. Comparatively, cellulose is a single substance. In contrast to cellulose, which is a linear polymer of -D glucose, amylose is a linear polymer of -D glucose. C1 – C4 – glycosidic bond is present in cellulose but not in amylose, which has the opposite structure. The structure of amylopectin is extensively branching.

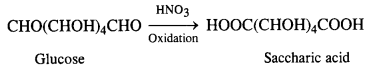
14.9 What happens when D-glucose is treated with the following reagents?
(i) HI
(ii) Bromine water
(iii) HNO3
Ans – (i) N-hexane is produced when D-glucose is heated with HI for an extended period of time. This demonstrates that the chain of six-carbon atoms is connected in a straight line.
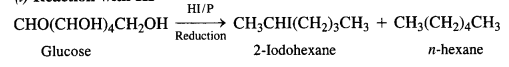
(ii) Reaction with bromine water

(iii) HnO3
14.10 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Ans – (1) Aldehydes produce the 2, 4 DNP test, Schiff’s test, and the hydrogen sulphite addition product when they interact with NaHSO4. Despite possessing aldehydes, glucose does not experience these reactions.
(2) The hydroxylamine does not react with glucose pentaacetate. This demonstrates that glucose does not include a free CHO group.
(3) There are two crystalline forms of glucose: and. At 303 K, a concentrated glucose solution crystallises into the -form (m. p. = 419 K), while at 371 K, a heated, saturated aqueous solution crystallises into the -form (m. p. = 423 K).
Because glucose has an open chain structure, this behaviour cannot be accounted for.
14.11 What are essential and non-essential amino acids? Give two examples of each type.
Ans – The human body needs essential amino acids, yet it is unable to synthesise them on its own. They must be consumed via meals. Leucine and valine are two examples.
The human body also needs non-essential amino acids, although it is also capable of synthesising them. Examples include glycine and alanine.
14.12 Define the following as related to proteins
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Ans – (i) Peptide Linkage: Proteins are a-amino acid polymers that are joined together by peptide bonds or other types of peptide linkage. Peptide linkage is a chemical reaction that creates an amide between the -COOH and -NH2 groups. The interaction of the amino group of one molecule with the carboxyl group of the other initiates the reaction between two molecules that contain the same or different amino acids. This eliminates a water molecule and creates the peptide bond -CO-NH-. Due to the fact that the reaction’s end product is made up of two amino acids, it is known as a dipeptide. For instance, the dipeptide glycylalanine is produced when the carboxyl group of glycine and the amino group of alanine join.

(ii) Primary Structure: Proteins may have one or more polypeptide chains. A protein’s fundamental structure is thought to be the particular sequence of amino acids that are joined together in each polypeptide to form the protein. A distinct protein is produced whenever this fundamental structure, or the arrangement of amine acids, is altered.
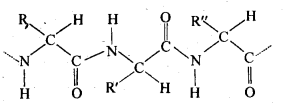
iii) Denaturation: Each protein in the biological system has a unique three-dimensional structure and has specific biologicalactivity. This is referred to as a protein’s natural form. Hydrogen bonds are destroyed when a protein is subjected to a physical change, such as a change in temperature, or a chemical change, such as a change in pH, etc. In order to produce fibrous proteins, which are insoluble in water, soluble forms of proteins, such as globular proteins, go through coagulation or precipitation. Additionally, this coagulation causes the proteins’ biological activity to decline, a process known as denaturation. Proteins’ 2° and 3° structures are damaged during denaturation, while their 1° structure is left untouched.
14.13 What are the common types of secondary structure of proteins?
Ans – Secondary structure of protein refers to the shape in which a long polypeptide chain can exist. These are found to exist in two types :
- α-helix structure
- β-pleated sheet structure.
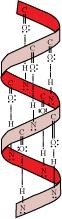
(a) α-Helix structure: If the size of the R-groups are quite large, the hydrogen bonding occurs between > C = O group\sof one amino acid unit and the > N-H group of the fourth amino acid unit within the same chain. In order to create this right-handed ct-helix structure, the polypeptide chain coils up. Most fibrous structural proteins, including those found in muscles, hair, and wool, adopt this type of structure. These proteins can be stretched because they are elastic. The weak hydrogen bonds that cause the a-helix are disrupted during this phase. This tends to make the helix grow longer like a spring. The hydrogen bonds are created again after the strain is released.
(b) Flat sheet or pleated sheet structure: If R-groups are short, the peptide chains are arranged zigzag-style, with the alternate R-groups on the same side spaced at specific intervals. Hydrogen bonds between the two such neighbouring chains keep them together. When several of these chains are interconnected, a flat sheet structure is created. To fit R-groups of average size, these chains may slightly contract or budge. A pleated sheet structure, also known as a pleated sheet, is created when the sheet bends into parallel folds. Then, to create a three-dimensional structure, these sheets are piled one on top of the other like book pages.
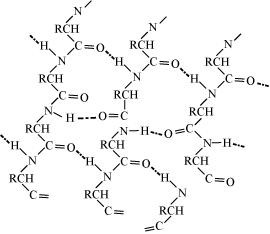
14.14 What type of bonding helps in stabilising the α-helix structure of proteins?
Ans – α-helix structure of proteins is stabilised through hydrogen bonding. Helix structure (a). The > C = O group of one amino acid unit and the > N- H group of the fourth amino acid unit within the same chain form a hydrogen bond when the R-group sizes are quite large. In order to create this right-handed a-helix structure, the polypeptide chain coils up. Most fibrous structural proteins, including those found in muscles, hair, and wool, adopt this type of structure. These proteins can be stretched because they are elastic. The weak hydrogen bonds that cause the -helix are disrupted during this procedure. This tends to make the helix grow longer like a spring.
14.15 Differentiate between globular and fibrous proteins.
Ans –
| Globular proteins | Fibrous proteins |
| 1. Polypeptide chains are arranged as coils. | 1.Polypeptide chains run parallel to each other. |
| 2. They have a spherical shape. | 2. They have a thread-like structure. |
| 3. These are water-soluble. | 3. These are insoluble in water. |
| 4. These are sensitive to a small change in temperature and pH. | 4. These are not affected by a small change in temperature and pH. |
| 5. They possess biological activity. | 5. They don’t have any biological activity but serve as the chief structural material of animal tissues |
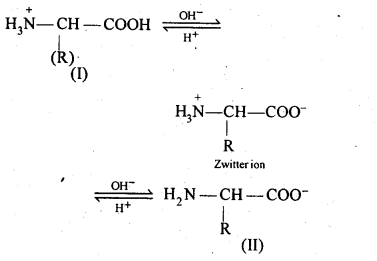
14.16 How do you explain the amphoteric behaviour of amino acids?
Ans – Amino acids are molecules that combine a basic (amino group) and an acidic (carboxyl group) group. They cancel out one another in an aqueous solution. While the amino group gains a proton, the carboxyl group loses one. A dipolar or zwitter ion is created as a result.
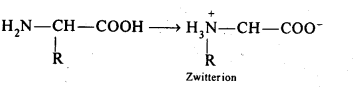
In zwitter ionjc form
14.17 What are enzymes?
Ans – Carbs are known as body fuels because they give the body the energy it needs to function, as we have seen through the study of carbohydrates. In reality, the human body functions much like a furnace where chemical reactions take place and are in charge of food digestion, molecule absorption, and energy creation. A number of processes are catalysed during the entire process by biocatalysts called enzymes. Enzymes can therefore be described as biological or biocatalysts that speed up chemical reactions in living things.
Enzymes are extremely specialised for a certain substrate and reaction. These are typically named after the compounds or group of substances that they interact with or work with.
14.18 What is the effect of denaturation on the structure of proteins?
Ans – A protein is discovered to have a distinct three-dimensional structure and a distinct biological activity in a biological system. This protein is thought to be its natural form. When a protein is subjected to a physical change (such as a change in temperature) or a chemical change (such as a change in pH), denaturation takes place and the hydrogen bonds in the protein are broken.
This disruption causes the protein molecule’s globules and helix to uncoil. The protein’s biological activity is reduced as a result. The protein’s secondary and tertiary structures are damaged during denaturation, while the fundamental structure is unaffected.
The globular proteins are changed into fibrous proteins as a result of denaturation. So, coagulation results from denaturation.
14.19 How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Ans – Vitamins are divided into two groups based on whether they are soluble in water or fat.
(i) Fat-soluble vitamins: These are vitamins that can dissolve in fat and oils but not in water. For instance: A, D, E, and K vitamins
(ii) Water-soluble vitamins: This category includes vitamins that can dissolve in water. B vitamins (B1, B2, B6, B12, etc.) and vitamin C are two examples.
Biotin, sometimes known as vitamin H, is not soluble in either water or fat.
The coagulation of blood is caused by vitamin K.
14.20 Why are vitamin A and vitamin C essential to us? Give their important sources.
Ans – Vitamin A is water soluble but insoluble in fats and oils. destroyed through cooking or extended air exposure. It strengthens the body’s defences against illness. keeps skin healthy and aids in the recovery of injuries and abrasions. It is offered in
Vitamin C is resilient to heat and soluble in oils and fats but insoluble in water. enhances vision and fosters growth. It also makes people more resistant to illness. It can be found in citrus fruits (oranges, lemons, grapefruit, limes, etc.), amia, cabbage, guava, and other produce.
14.21 What are nucleic acids? Mention their two important functions.
Ans – As one of the components of chromosomes, nucleic acids are macromolecules that may be found in the nuclei of all live cells. There are two types of nucleic acids:
(i) Deoxyribonucleic acid (DNA)
(ii) Ribonucleic acid (RNA)
Nucleic acids are also known as polynucleotides as they are long-chain polymers of nucleotides. Two important functions of nucleic acids are:
(i)Heredity is determined by DNA. It is the process of passing on innate characteristics to the following generation.
(ii) The synthesis of proteins in a cell is carried out by nucleic acids, including DNA and RNA. These are essential for the development and upkeep of our body. Although the numerous RNA molecules in a cell actually produce the proteins, a DNA molecule provides the signal for a specific protein to be synthesised.
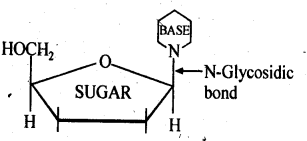
14.22 What is the difference between a nucleoside and a nucleotide?
Ans – A pentose sugar and a nitrogenous base are the only two fundamental parts of nucleic acids that make up a nucleoside. It is created when a pyrimidine base at position 1 (cytosine, thiamine, or uracil) or a purine base at position 9 (guanine, adenine, or uracil) is linked to a sugar at position 1 (ribose or deoxyribose) by a -linkage. Since a nucleotide is the repeating structural component of nucleic acids, they are also known as polynucleotides.
All three of the fundamental elements of nucleic acids—a phosphoric acid group, a pentose sugar, and a nitrogenous base—are present in a nucleotide. These are produced by esterifying the pentose sugar’s C5, -OH group with phosphoric acid.
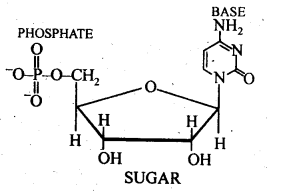
14.23 The two strands in DNA are not identical but are complementary. Explain.
Ans – The two strands of DNA’s helical helix are joined by hydrogen bonds between particular base pairs. While thymine makes a hydrogen bond with adenine, cytosine forms one with guanine. The two strands are so complementary to one another as a result. Additionally, the bases in one strand’s sequence naturally determine those in the other.
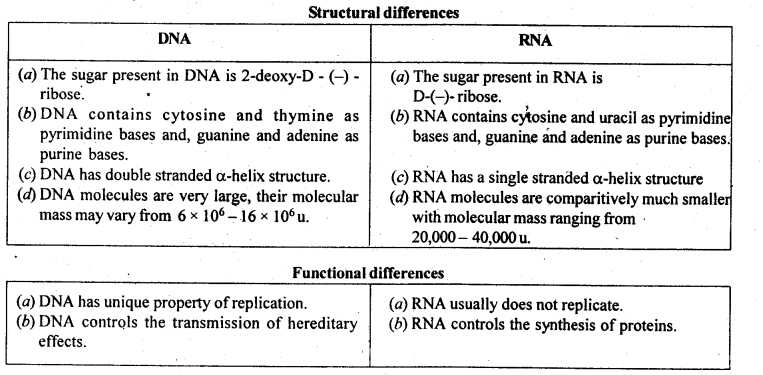
14.24 Write the important structural and functional differences between DNA and RNA.
Ans –
14.25 What are the different types of RNA found in the cell?
Ans – There are several types of RNA in a cell, including:
(i) Messenger RNA (m-RNA):
(ii) Messenger RNA (m-RNA): The DNA produces m-RNA, which is single stranded and makes up roughly 15% of all RNA.
(iii) Ribosomal RNA (r-RNA): The ribosomes contain ribosomal RNA. In order to produce ribosomes, it is typically combined with protein. DNA uses the nucleus to synthesise it. The majority of total RNA, or roughly 80%, is single stranded. It is stable metabolically.
(iv) Transfer RNA (t-RNA): DNA synthesises transfer RNA in the nucleus. It is single stranded and soluble RNA. It makes up around 5% of all RNA and has a very brief lifespan.




