NCERT Solved Exercise Questions – Class 11 Biology Chapter 9 Biomolecules
9.1 What are macromolecules? Give examples.
Ans – There are two types of biomolecules, or chemical compounds, found in living things. One is that the molecules with molecular weights under 1,000 are commonly referred to as macromolecules or just biomolecules, whereas the molecules discovered in the acid-insoluble fraction are referred to as macromolecules or biomacromolecules.
With the exception of lipids, the molecules in the insoluble fraction are made of polymeric materials. Why, then, do lipids—whose molecular weights are less than 800—belong to the macromolecular fractions of acid-insoluble fractions?
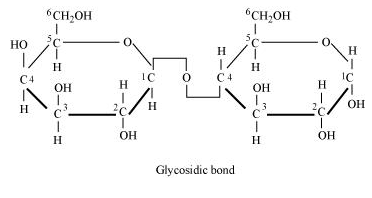
9.2 Illustrate a glycosidic, peptide and a phospho-diester bond.
Ans – (a) A glycosidic link forms when two monosaccharide molecules in a polysaccharide come together. Two neighbouring monosaccharides’ two carbon atoms join together to form this connection.
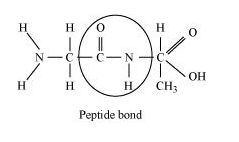
(b) Peptide bond: A peptide bond connects two amino acids by connecting the carboxyl (- COOH) group of one and the amino (- NH2) group of the following amino acid, which is created during the dehydration process.
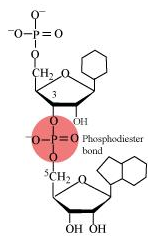
(c) Phosphodiester bond: This is the ester link that connects the phosphate with the sugar’s hydroxyl group. It is known as a phosphodiester bond since this ester bond is present on both sides.
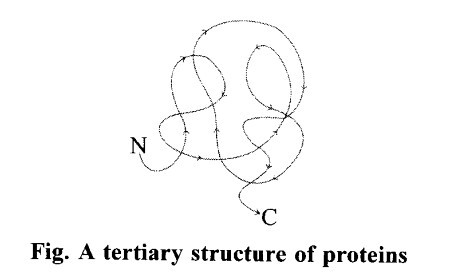
9.3 What is meant by tertiary structure of proteins?
Ans – The helical polypeptide molecule can fold on itself and take on a complicated but distinct shape, such as a sphere, a rod, or any shape in between. These geometrical patterns are referred to as the protein molecules’ tertiary (3°) structure. The polypeptide molecules’ coils and folds are structured so that the polar side chains are exposed and the non-polar amino acid chains are hidden inside. To create the active regions of enzymatic proteins, a protein’s tertiary structure draws far-off amino acid side chains closer together.
Weak bonds formed between two parts of a polypeptide, such as hydrogen, ionic, disulphide, and hydrophilic-hydrophobic bonds, keep the tertiary structure in place. The function of proteins is easily interrupted by changes in pH, temperature, and chemicals.
9.4 Find and write down structures of 10 interesting small molecular weight biomolecules. Find if there is any industry which manufactures the compounds by isolation. Find out who are the buyers.
Ans – Minerals (such as salt, potassium, calcium, zinc, iodine, etc.), gases (such as ozone, n2, c02, nh3), sugars (ribose, deoxyribose, glucose, fructose), lipids, amino acids, and nucleotides are among the interesting small molecular weight biomolecules (pyrimidines & purine). Following are the structures of 10 intriguing small-molecular-weight biomolecules:
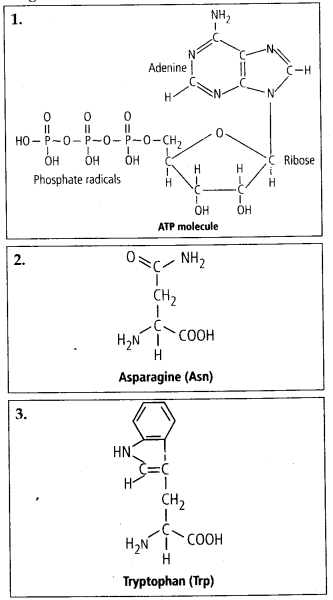
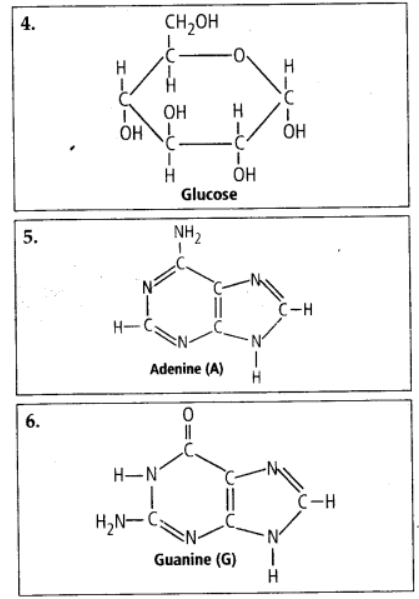
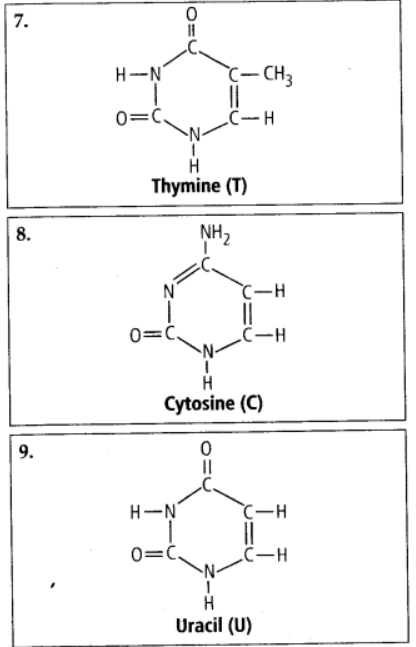
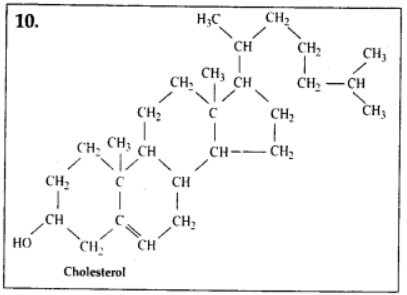
9.5 Proteins have primary structure. If you are given a method to know which amino acid is at either of the two termini (ends) of a protein, can you connect this information to purity or homogeneity of a protein?
Ans – Several scientists have offered a variety of approaches for determining the order of amino acids. In order to determine the amino acid sequence in a polypeptide chain, Frederick Sanger proposed Sanger’s reagent.
Sanger used 1-fluoro 2, 4-dinitrobenzene (FD NB) to analyse the structure of the hormone insulin. To create a dinitrophenyl (DNP) derivative of peptide, FDNB specifically binds to the N-terminal amino acid. Chromatography can be used to determine the identity of this DNP-derived peptide. The homogeneity of a protein molecule is demonstrated by the identified amino acid sequence.
9.6 Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g., Cosmetics etc.)
Ans – Thrombin, fibrinogen, enkephalins, antigens, antibodies, streptokinase, protein tyrosine kinase, diastase, renin, insulin, oxytocin, vasopressin, and other proteins are utilised as therapeutic agents. Additionally, proteins are employed in biological buffers, research methodologies, the dairy and textile industries, and cosmetics.
9.7 Explain the composition of triglyceride.
Ans – They are frequently referred to as neutral fats since they are non-polar in nature and insoluble in water. Unlike carbohydrates, which have a much greater ratio of carbon to oxygen atoms, neutral or depot fats are made of carbon, hydrogen, and oxygen.
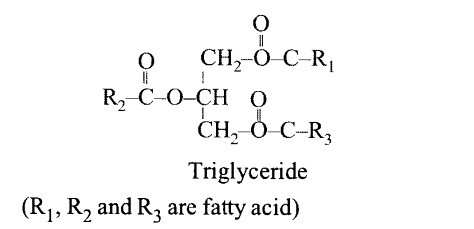
(i) Glycerol: A molecule of glycerol has three carbons, each of which is home to a hydroxyl (-OH) group.
(ii) Fatty acids – Each carbon atom (C) forms four bonds to other atoms in a fatty acid molecule, which is an unbranched chain of carbon atoms. At one end, it has a carboxyl group called COOH, and a hydrogen atom (H) is linked to all or almost all of the carbon atoms to form a hydrogen chain. Non-polar bonds between carbon and hydrogen exist. The hydrocarbon chain does not dissolve in water as a result.
9.8 Can you describe what happens when milk is converted into curd or yoghurt, from your understanding of proteins.
Ans – Casein, a milk protein, is broken down during the curdling process of milk. Curd is semi-digested milk. Renin transforms milk protein into calcium paracaseinate, also known as the curd or yoghurt, in the stomach by reacting with Ca++ ions.
9.9 Can you attempt building models of biomolecules using commercially available atomic models (Ball and Stick models).
Ans – Yes, atomic models that are available commercially can be used to create models of biomolecules.
When displaying the structure of chemical substances or biomolecules, 3D or spatial molecular models such as ball and stick and space filling models are used. In the ball and stick model, covalent bonds are represented by the straight lines that connect the atoms’ centres. Double and triple bonds are frequently portrayed by springs that curvely bind the balls together. While the space occupied by the atoms is either not represented at all or is primarily indicated by the relative sizes of the spheres, bond angles and bond lengths reflect the actual relationships.
9.10 Attempt titrating an amino acid against a weak base and discover the number of dissociating ( ionizable ) functional groups in the amino acid.
Ans – An amino acid’s COOH group also functions as a weak acid when titrated with a weak base. As a result, a salt with a weak base forms, and the resulting solution’s pH is close to 7, therefore there is no abrupt change. There are just two dissociating functional groups: an amino group (NH2) and a carboxylic group ( – COOH). Amino acid serves as an indication during the titration. When necessary, amino acids in a solution take on a basic or acidic behaviour. This is why they are also known as amphipathic molecules.
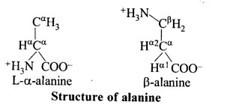
9.11 Draw the structure of the amino acid, alanine.
Ans –
9.12 What are gums made of? Is Fevicol different?
Ans – Gums can be divided into biomolecules or secondary metabolites. Numerous substances are found in plant, fungal, and microbial cells. They come from these sources. nonetheless, is distinct. Fevicol is not created from cells that were written on paper.
9.13 Find out a qualitative test for proteins, fats and oils, amino acids and test any fruit juice, saliva, sweat and urine for them.
Ans – Tests of quality for lipids, proteins, and amino acids:
The biuret test detects the presence of proteins by turning the solution violet in colour. In a basic solution, biuret H2NCONHCONH2 interacts with copper ions to produce violet colour.
Lipoprotein Liebermann-Burchard Test:
This is a blend of sulfuric acid and acidic anhydride. When combined with cholesterol, this produces a green colour.
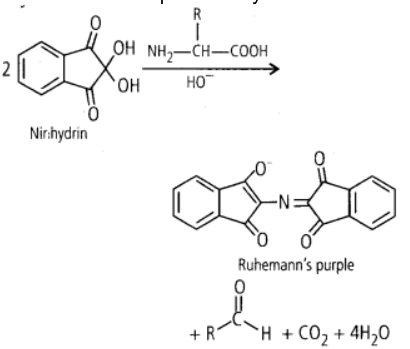
The ninhydrin (2,2 Dihydroxy indane-l,3-dione) chemical is used to identify ammonia as well as primary and secondary amines. Ruhemann’s purple, a deep blue or purple colour, is created when these free amines react. Ninhydrine also analyses the amino acids in proteins. Except for proline, the majority of amino acids, including a-amino acids, are hydrolyzed and react with ninhydrin (a secondary amine). A coloured substance is created when ninhydrin combines with an amino acid that also has a free amino group and a free carboxylic acid group. The condensation product is yellow when the amino group is secondary.
Oils and fats can be tested for solubility by seeing if they dissolve in lighter fluid rather than water. In this experiment, two test tubes holding 10 drops each of lighter fluid and cold water are added 5 drops of fat or oil.
Fruit juice contains sugar, hence the tests stated above cannot be used to evaluate it. Saliva can be analysed for proteins and amino acids because it contains proteins, mineral salts, amylase, etc. Proteins can be detected in urine because it contains them.
9.14 Find out how much cellulose is made by all the plants in the biosphere and compare it with how much of paper is manufactured by man and hence what is the consumption of plant material by man annually. What a loss of vegetation!
Ans – A 2006 UN research estimates that just the biomass of trees contains 312 billion tonnes of carbon. The amount rises to nearly 1.1 trillion tonnes of carbon when you factor in the carbon found in deadwood, litter, and forest soil! The UN assessment also reveals that the loss of forests results in an annual increase in carbon dioxide emissions of nearly 2.2 billion tons, or about as much as the United States emits. Many climate experts think that one of the best and least expensive ways to combat climate change is through the preservation and restoration of forests.
Although it is challenging to obtain precise information, the information above can provide a general estimate of the amount of cellulose produced by plants. In order to make paper, about 10% of cellulose is used. The issue is worsened by incorrect wood-cutting and replanting practises despite the lower percentage. Older trees are typically felled for enormous amounts of cellulose, and only a few types of plants can be replanted. Certain species disrupt biodiversity because they promote monoculture.
The issue becomes even more problematic when effluents from a paper industry are added.
9.15 Describe the important properties of enzymes.
Ans – The following are some of enzymes’ crucial characteristics:
(i) The enzymes are typically complex globular proteins with a large molecular weight. For their activity, they can collaborate with non-protein substances.
(ii) Enzymes simply speed up chemical reactions; they do not initiate them. They just briefly mix with the molecules of the substrate and are not permanently consumed or altered by the reaction they catalyse.
(iii) Reversible enzyme-controlled processes.
(iv) The enzymes work in a certain way. An enzyme exclusively acts on a certain substrate or catalyses a specific type of reaction.
(v) The enzymes can only work at an ideal temperature because they are thermolabile, or heat sensitive. Similar to this, enzyme activity is highest at the ideal pH.
(vi) Poisons render the enzymes inactive.




