NCERT Exercise Solutions – Chemistry Chapter 15 Polymer
15.1 Explain the terms polymer and monomer.
Ans – High molecular mass macromolecules called polymers are created by repeating structural elements called monomers. Polymers typically have a molecular mass between 103 and 107 u. A polymer is made up of different monomer units.
covalent connections that are very strong. Both manufactured and natural polymers are possible. Buna-N, rubber, Bakelite, and polyethylene are a few examples of polymers. Simple, receptive molecules known as monomers connect with one another in massive covalent bonding in chains to produce polymers.
Styrene, vinyl chloride, ethene, and propene are a few examples of monomers.
15.2 What are natural and synthetic polymers? Give two examples of each type.
Ans – 1. Natural polymers: These are the polymers that are found naturally, primarily in plants and animals. Starch, cellulose, proteins, rubber nucleic acids, and other substances are a few typical examples. Among these, the polymers of glucose molecules are starch and cellulose. Amino acids, which can be connected in various ways, are the building blocks of proteins. Unit 15 on biomolecules has a detailed discussion of them. Another important polymer that is made from the latex of the rubber tree is natural rubber. The unsaturated hydrocarbon 2-methyl-i, 3-butadiene, often known as isoprene, serves as the basis for the monomer units.
Natural rubber, cellulose, nucleic acids, proteins, etc. are examples of natural polymers.
2. Synthetic polymers: These are the kind of polymers that are created in a laboratory. These are also known as man-made polymers and were created in the 20th century to suit the modern civilisation’s ever-increasing need.
Dacron (or terylene), Bakelite, PVC, Nylon-66, Nylon-6, and other examples of synthetic polymers
15.3 Distinguish between the terms homopolymer and copolymer and give an example of each.
Ans –
| Homopolymer | Copolymer |
| The polymers that are formed by the polymerization of a single monomer are known as homopolymers. In other words, the repeating units of homopolymers are derived only from one monomer. For example, polythene is a homopolymer of ethene. | The polymers whose repeating units are derived from two types of monomers are known as copolymers. For example, Buna−S is a copolymer of 1, 3-butadiene and styrene. |
15.4 How do you explain the functionality of a monomer?
Ans – Functionality of a monomer refers to the quantity of binding sites that are present in that monomer so as to bond with other monomers to form a polymer.
Ethene and propene, for instance, have one functionality while 1, 3-butadiene and adipic acid have two.
15.5 Define the term polymerisation.
Ans – The process of polymerization involves the chemical reaction between monomer molecules to create lengthy polymer chains or three-dimensional networks. Different monomer units are linked together by powerful covalent bonds to form polymers.
Two general categories can be used to classify polymerization:
1. Addition or Chain-Growth Polymerization
2. Condensation or Step-Growth Polymerization
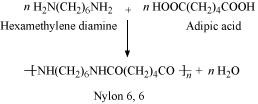
An illustration of a polymerization reaction is the reaction that occurs when hexamethylenediamine and adipic acid mix to produce nylon 6,6
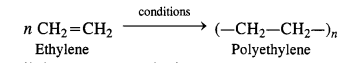
15.6 Is ( NH-CHR-CO ) n , a homopolymer or copolymer?
Ans – It is a homopolymer because the repeating structural unit, NH2—CHR—COOH, only contains one type of monomer.
15.7 Why do elastomers possess elastic properties?
Ans – Polymers are classified into four classes on the basis of molecular forces. Elastomers, fibres, thermoplastic polymers, and thermosetting polymers fall under this category.
1. Elastomers: These polymers have the least intermolecular forces. Because of this, they are easily stretched by minor stresses and quickly return to their original shape when the stresses are released. The addition of certain cross-links to the polymer chains can further boost the elasticity. In terms of elastomers, natural rubber is the most well-known example. The materials buna-S, buna-N, and neoprene are a few additional examples.
2. Fibres: Fibres are a type of polymer that resembles thread and may be woven in various ways to create fabrics. These are frequently used to create items like clothing, nets, ropes, and gauze. Because the chains within the fibres have strong intermolecular forces like hydrogen bonds, the fibres have a high tensile strength. The close packing of the chains is also a result of these forces. The fibres are therefore crystalline in form and have extremely high melting temperatures. Among the more popular polymers in this class include nylon 66, terylene, and polyacrylonitrile, among others.
3. Thermoplastics: These are linear polymers with weak van der Waals forces acting in their various chains, which are an intermediary force between that found in elastomers and that found in fibres. They may be formed into various shapes by using the appropriate moulds because when heated, they melt and create a fluid that solidifies into a hard mass when cooled. Polyethylene, polystyrene, polyvinyls, etc. are only a few typical examples. These can be used to create buckets, toys, telephone equipment, television cabinets, and other items.
4. Thermosetting plastics: These are often low-molecular-mass, semifluid compounds. They become rigid and infusible when heated because of the cross-linking of the polymer chains. They consequently take on a three-dimensional quality.
15.8 How can you differentiate between addition and condensation polymerisation?
Ans – In contrast to polymerization, which eliminates tiny molecules like water and NH3, adding together the molecules of the same or distinct monomers results in the development of macromolecules.
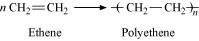
In most cases, addition polymerization happens between molecules with double and triple bonds. Creating polythene from ethene and neoprene from chloroprene, for instance, as well as other examples With the removal of simple molecules like water, alcohol, ammonia, carbon dioxide, and hydrogen chloride, two or more bifunctional, trifunctional molecules go through a sequence of separate condensation processes to generate a macromolecule. For instance, nylon-6,6 is a condensation polymer created by removing water molecules from hexamethylenediamine and adipic acid.
15.9 Explain the term copolymerisation and give two examples.
Ans – A polymer that is created from more than one kind of monomer is called a copolymer.
Copolymerization is the process of polymerizing monomers into copolymers.
In the copolymer, each monomer is present in many units.
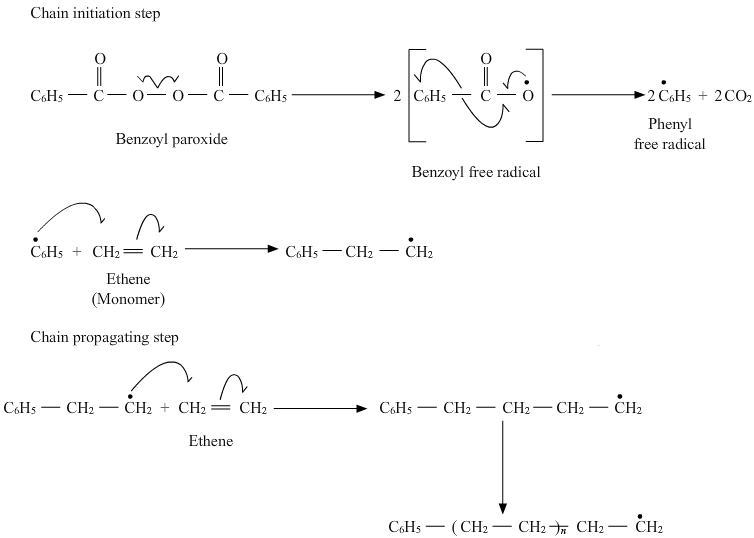
15.10 Write the free radical mechanism for the polymerisation of ethene.
Ans – The polymerisation of ethene to polythene consists of heating or exposing to light a mixture of ethene with a small amount of benzoyl peroxide initiator The phenyl free radical produced by the peroxide is added to the ethene double bond to begin the process, creating a new and bigger free radical. The chain beginning step is this action. Another radical with a larger size is created when this radical interacts with a second ethene molecule. The reaction progresses when this cycle is repeated with new, stronger radicals, and this phase is known as a chain propagating step. In the end, the radical created by this process reacts with another radical to create the polymerized product. The chain termination step is what we refer to as here.
15.11 Define thermoplastics and thermosetting polymers with two examples of each.
Ans – Thermoplastic polymers are long-chain, linear (slightly branched) polymers that can repeatedly become softer when heated and harder when cooled. As a result, they can be changed repeatedly. They have intermolecular forces of attraction that are between elastomers and fibres in strength. Thermoplastics include things like polyvinyl, polystyrene, and polythene.
Thermosetting polymers are strongly branched and cross-linked polymers that, when heated, extensively cross-link in moulds and become infusible. On heating, these polymers cannot be re-softened. Bakelite and urea-formaldehyde resins are two examples of thermosetting plastics.
15.12 Write the monomers used for getting the following polymers.
(i) Polyvinyl chloride
(ii) Teflon
(iii) Bakelite
Ans –
- vinyl chloride
(CH2=CHCl)
- Tetrafluoroethylene
( CF2 = CF2)
- phenol and formaldehyde.

15.13 Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Ans – One common initiator used in free radical addition polymerization is benzoyl peroxide.
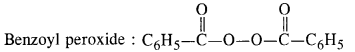
15.14 How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Ans – Natural rubber is made of 1, 4-polymerized isoprene units and is cis-polyisoprene. Each isoprene unit in this polymer has a double bond between the carbon atoms C2 and C3. Intermolecular forces are rather modest because these cis-double bonds prevent the polymer chains from interacting effectively by allowing them to move closer to one another. Natural rubber, or cis-polyisoprene, has a randomly coiled structure rather than a linear one as a result, and it exhibits elasticity as a result.
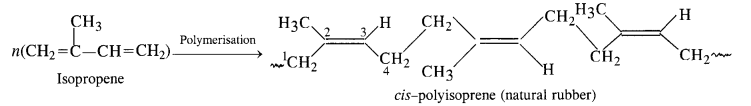
Because polar groups are absent and the double bonds’ cis-configurations prevent the polymer chains from entangling, natural rubber has a high elasticity. Only weak van der Waals forces are therefore present. The chains have elastic qualities and can be stretched like springs because they are not linear.
15.15 Discuss the main purpose of vulcanisation of rubber.
Ans – Natural rubber is vulcanised to increase its tensile strength, hardness, and other qualities. A mixture of raw rubber, sulphur, and the proper additives is heated during vulcanization at a temperature between 373 K and 415 K. The process is sluggish, thus some additives, such zinc oxide, are employed to speed it up. Sulfur cross-links are created during this process, increasing the tensile strength while increasing rubber’s hardness and toughness.
Excellent flexibility, low water absorption, and resistance to oxidation and organic solvents are all characteristics of this vulcanised rubber.
15.16 What are the monomeric repeating units of Nylon-6 and Nylon-6,6?
Ans – Nylon 6’s [NH – (CH2)5 – CO] monomeric repeating unit is generated from caprolactam.
The monomeric repeating unit of nylon 6, 6, which is generated from hexamethylene diamine and adipic acid, is [NH – (CH2)6 – NH – CO – (CH2)4 – CO].
15.17 Write the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Buna-N
(iii) Dacron
(iv) Neoprene
Ans –

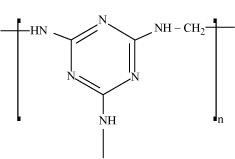
15.18 Identify the monomer in the following polymeric structures.
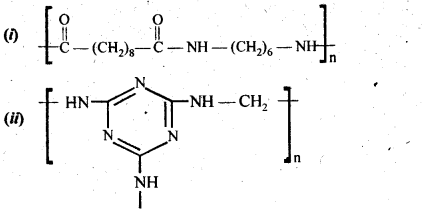
Ans – (i) Decanedioic acid: HOOC(CH2)8COOH
Octamethylenediamine: H2N(CH2)8NH2
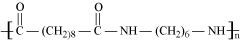
(ii)
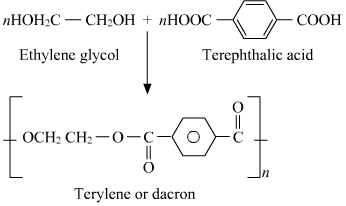
15.19 How is dacron obtained from ethylene glycol and terephthalic acid ?
Ans – By condensation polymerizing ethylene glycol and terephthalic acid while removing water molecules, dacron is produced. An antimony trioxide and zinc acetate mixture serves as the catalyst for the reaction, which takes place between 420 and 460 K.
15.20 What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
Ans – A biodegradable polymer is one that can be broken down by microbes.
When biodegradable polymers are broken down by living creatures, they break down into simpler elements like water, carbon dioxide, nitrogen, etc.
An illustration of a biodegradable aliphatic polyester is polyhydroxybutyrate/cohydroxyvalerate (PHBV).





