Chapter 12 Aldehydes , Ketones and Carboxylic Acids Notes
12.1 Write the structures of the following compounds.
(i) α-Methoxypropionaldehyde (ii) 3-Hydroxybutanal
(iii) 2-Hydroxycyclopentane carbaldehyde (iv) 4-Oxopentanal
(v) Di-sec. butyl ketone (vi) 4-Fluoroacetophenone
Ans: (i)
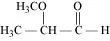
(ii)
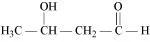
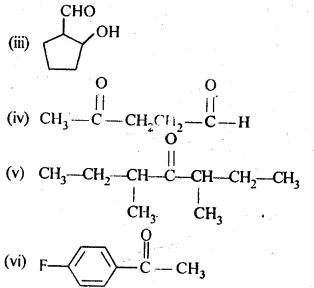
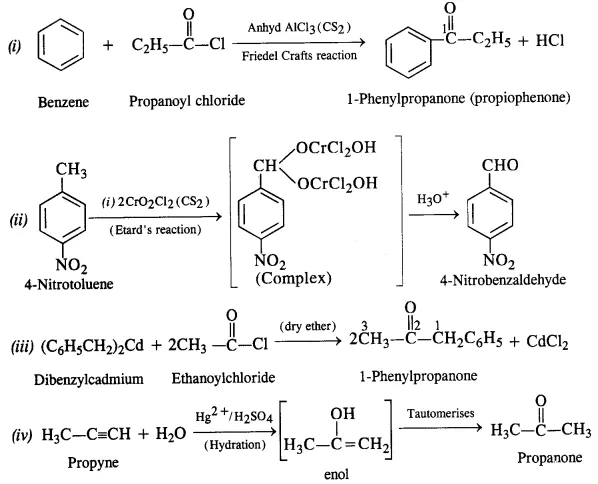
12.2 Write the structures of products of the following reactions:
Ans:
12.3 Arrange the following compounds in increasing order of their boiling points. CH3CHO, CH3CH2OH, CH3OCH3 , CH3CH2CH3
Ans: The increasing order of boiling points of all these compounds of comparable molecular masses is :
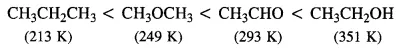
The molecular masses of the given compounds are in the range 44 to 46. CH3CH2OH undergoes extensive intermolecular H-bonding, resulting in the association of molecules. Therefore, it has the highest boiling point. CH3CHO is more polar than CH3OCH3 and so CH3CHO has stronger intermolecular dipole − dipole attraction than CH3OCH3⋅
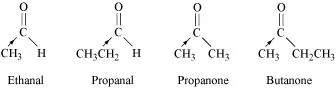
12.4 Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
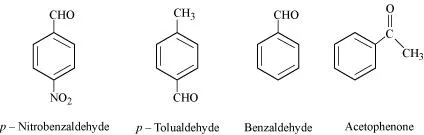
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint: Consider steric effect and electronic effect.
Ans: (i) Butanone < Propanone < Propanal < Ethanal
The electron density at the carbonyl carbon increases with the increase in the +I effect.
As a result, the chances of attack by a nucleophile decrease.
(ii)
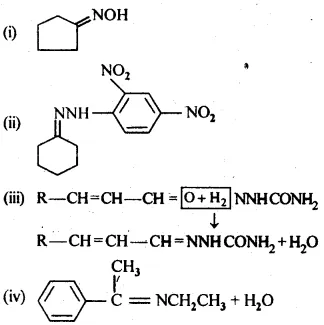
12.5 Predict the products of the following reactions:
Ans:
12.6 Give the IUPAC names of the following compounds:
Ans: (i) 3-Phenylpropanoic acid
(ii) 3-Methylbut-2-enoic acid
(iii) 2-Methylcyclopentanecarboxylic acid
(iv) 2,4,6-Trinitrobenzoic acid
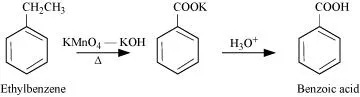
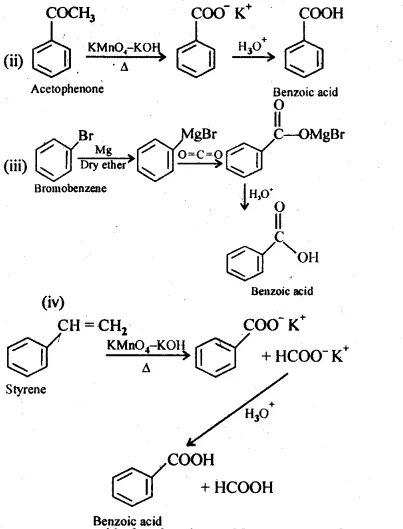
12.7 Show how each of the following compounds can be converted to benzoic acid.
(i) Ethylbenzene (ii) Acetophenone
(iii) Bromobenzene (iv) Phenylethene (Styrene)
Ans: (i)
12.8 Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or FCH2CO2H
(ii) FCH2CO2H or ClCH2CO2H
(iii) FCH2CH2CH2CO2H or CH3CH(F)CH2CO2H
Ans: CH3 group with +I effect increases the electron density on the oxygen atom in O – H bond in the carboxyl group and cleavage of bond becomes diffcult. It therefore, decreases the acidic strength.
- H2CFCOOH will be stronger of the two. The presence of electronegative F atom at the α-C causes electron withdrawal from the COOH and facilitates the release of H+.
- CH2FCO2H is a stronger acid for the same reason as stated above. F is more electronegative than Cl, so it withdraws electrons from the carboxyl group to a greater extent.CH3CHFCH2COOH is stronger. Although both the given acids have F atom in them, it is the proximity of F in CH3CHFCH2COOH to the COOH group which makes it more acidic.

