Chapter 5 Surface Chemistry Class 12 Chemistry Notes for CBSE Students
5.1 Write any two characteristics of Chemisorption.
Ans: The two characteristics of Chemisorption are:
- In Chemisorption which is highly specific in nature, the adsorbate and adsorbent get attached by chemical bonds which are either covalent or ionic in nature.
- High activation energy is required and high temperature is also favourable.
- Chemisorption increases with the increase in surface area which results in more number of active sites.
5.2 Why does physisorption decrease with the increase of temperature?
Ans: As Physisorption is Exothermic in nature, which means when gas gets adsorbed on the solid surface, Heat is evolved.
So, according to Le-Chatelieres when the temperature is an increased reverse process (Desorption) will be favoured. So, Physisorption decreases with the increase of temperature.
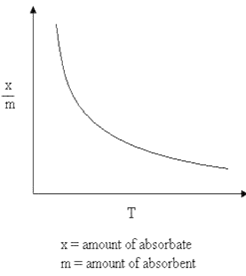
Where x/m: Volume of gas adsorbed T: Temperature
5.3 Why are powdered substances more effective adsorbents than their crystalline forms?
Ans: Since adsorption is a surface phenomenon. So, the extent of adsorption directly depends on the surface area.
Thus, the powdered substances more effective absorbents than their crystalline forms due to their large surface area which can adsorb more gas as compared to the crystalline form.
5.4 In Haber’s process, hydrogen is obtained by reacting methane with steam in presence of NiO as catalyst. The process is known as steam reforming. Why is it necessary to remove CO when ammonia is obtained by Haber’s process?
Ans: It is important to remove CO (Carbon Monoxide) in the synthesis of ammonia as CO affects the activity of Iron catalyst which is required in Haber’s process.
CO acts as a poison for the catalyst used in the manufacture of NH3 by Haber’s process. Hence, it is necessary to remove it.
5.5 Why is the ester hydrolysis slow in the beginning and becomes faster after sometime?
Ans: The ester hydrolysis takes place as follows:
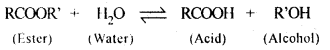
The acid produced in the reaction acts as an auto catalyst for the reaction. Hence, the reaction becomes faster after some time.
5.6 What is the role of desorption in the process of catalysis.
Ans: In catalytic reaction, the reactant attached on the surface of the catalyst and form complex and after some procedure, it becomes the product of the reaction.
We need to detach it from the surface of the catalyst to get the product and catalyst separately and hereby desorption process, we can get the product.
5.7 What modification can you suggest in the Hardy Schulze law?
Ans: According to Hardy Schulze law, the coagulating ion has charge opposite to that on the colloidal particles. Hence, the charge on colloidal particles is neutralized and coagulation occurs. The modification to this law is :
When oppositely charged sols are mixed in proper proportions to neutralize the charges of each other, coagulation of both the sol occurs.
5.8 Why is it essential to wash the precipitate with water before estimating it quantitatively?
Ans: The precipitate which is obtained from a chemical reaction always contain some unwanted substances (eg ions, impurities) which get adsorbed onto the surface of the precipitate.
Therefore, it becomes important to wash the precipitate before estimating it quantitatively so as to remove these unwanted adsorbed substances and obtain accurate results.
