Chapter 7 The p-Block Elements Class 12 Chemistry Notes for CBSE Students
7.1 Why are pentahalides of P, As, Sb and Bi more covalent than their trihalides?
Ans: Pentahalides are more covalent than trihalides. This is due to the fact that in pentahalides +5 oxidation state exists while in the case of trihalides +3 oxidation state exists. So, Higher the +ve O.S of the central atom more will be the polarising power and more will be the covalent character in the bond between the central atom and a halogen atom. Since elements in +5 oxidation state will have more polarising power than in +3 oxidation state, the covalent character of bonds is more in pentahalides.
7.2 Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements?
Ans: Down the group, the atomic size of the element (E) increases and the bond length of the corresponding E—H bond also increases. This adversely affects the bond dissociation enthalpy. This means that amongst the trihydrides of the members of nitrogen family, the bond dissociation enthalpy of Bi—H bond is the least. Therefore, BiH3 is the strongest reducing agent among the hydrides of group 15 elements.
7.3 Why is N2 less reactive at room temperature?
Ans: Because of strong pπ – pπ overlap resulting into the triple bond. N = N due to which the bond dissociation energy of N2 is very high rendering it less reactive.
7.4 Mention the conditions required to maximise the yield of ammonia.
Ans: Ammonia is produced by Haber’s process-
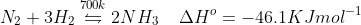
The maximum yield conditions for the production of ammonia are-
- Pressure = 200 atm or 200 x 105Pa
- Temperature of around 700 K
- Catalyst = Iron oxide
- Promotor = small amounts of K2O and Al2O3 to increase the rate of attainment of equilibrium.
7.5 How does ammonia react with a solution of Cu2+?
Ans: NH3 in the form of solution reacts with Cu2+ to form a complex with deep blue colour.
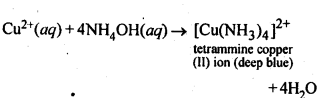
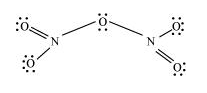
7.6 What is the covalence of nitrogen in N2O5?
Ans: We can see From the structure of N2O5 that covalence of nitrogen is four
7.7 Bond angle in PH4 + is higher than that in PH3. Why?
Ans: Both are sp3 hybridised.
In PH4+ all the four orbitals are bonded whereas in PH3 there is a lone pair of electrons on P, which is responsible for lone pair-bond pair repulsion in PH3 reducing the bond angle to less than 109° 28′.
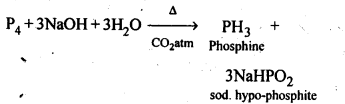
7.8 What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
Ans: When white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2, phosphine gas is liberated.
7.9 What happens when PCl5 is heated?
Ans: When we heat PCl5, it sublimes but decomposes on stronger heating and phosphorus trichloride is formed.

7.10 Write a balanced equation for the reaction of PCl5 with water.
Ans: PCl5 + D2O → POCl3, + 2DCl
7.11 What is the basicity of H3PO4?
Ans:
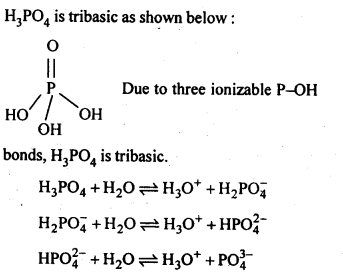
7.12 What happens when H3PO3 is heated?
Ans: On heating phosphorous acid dispropotionates to give orthophosphoric acid and phosphine.
4H3PO3 → 3H3PO4 + PH3
7.13 List the important sources of sulphur.
Ans: Combined sulphur exists as sulphates, such as gypsum, epsom, baryte and sulphides such as galena, zinc blende, copper pyrites, etc. Traces of sulphur occur as hydrogen sulphide in volcanoes.
Few organic materials like eggs, proteins, garlic, onion, mustard, hair and wool contain sulphur. 0.03 – 0.1% sulphur is present in the earth’s crust
7.14 Write the order of thermal stability of the hydrides of Group 16 elements.
Ans: The thermal stability of hydrides of group 16 elements decreases down the group. This is because down the group, size of the element (M) increases, M-H bond length increases and thus, stability of M-H bond decreases so that it can be broken down easily. Hence, we have order of thermal stability as H2O > H2S > H2Se > H2Te > H2PQ
7.15 Why is H2O a liquid and H2S a gas?
Ans: Due to high electronegativity of O than S, H2O undergoes extensive intermolecular H-bonding. As a result, H2O exists as an associated molecule in which each O is tetrahedrally surrounded by four H2O molecules. Therefore, H2O is a liquid at room temperature.
On the other hand,H2S does not undergo H- bonding. It exists as discrete molecules which are held together by weak van der waals forces of attraction. A small amount of energy is required to break these forces of attraction. Therefore, H2S is a gas at room temperature.
7.16 Which of the following does not react with oxygen directly? (Zn, Ti, Pt, Fe)
Ans: Since Platinum(Pt) is a noble metal .it will not react with oxygen directly
7.17 Complete the following reactions: (i) C2H4 + O2 → (ii) 4Al + 3 O2
Ans:
- C2H4 + 3O2 → 2CO2 + 2H2O
- 4Al + 3O2 → 2Al2O3
7.18 Why does O3 act as a powerful oxidising agent?
Ans: Due to the ease with which ozone liberates nascent oxygen atoms, it acts as a powerful oxidising agent.
O3 → O2 + O
7.19 How is O3 estimated quantitatively?
Ans: When O3 is treated with excess of KI solution buffered with borate buffer (pH = 9.2), I2 is liberated quantitatively.
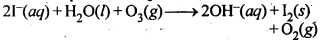
The I2 thus liberated is titrated against a standard solution of sodium thiosulphate using starch as an indicator
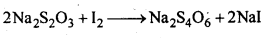
7.20 What happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
Ans: When sulphur dioxide is passed through an aqueous solution of ferric ions, ferric ions are reduced to ferrous ions.
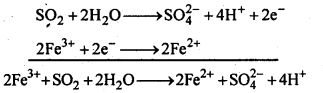
7.21 Comment on the nature of two S–O bonds formed in SO2 molecule. Are the two S–O bonds in this molecule equal?
Ans: SO2 exists as an angular molecule with OSO bond angle of 119.5°. It a resonance hybrid of two canonical-forms:
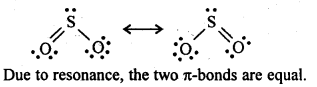
7.22 How is the presence of SO2 detected?
Ans: Presence of SO2 is detected by bringing a paper dipped in acidified potassium dichromate near the gas. If the paper turns green, it shows the presence of SO2 gas.
Or
It can be detected with the help of potassium permanganate solution. When SO2 is passed through an acidified potassium permanganate solution, it decolonizes the solution.
7.23 Mention three areas in which H2SO4 plays an important role.
Ans: Sulphuric acid is an important industrial chemical and is used for a lot of purposes. Some important uses of sulphuric acid are given below.
- It is used in fertilizer industry. It is used to make various fertilizers such as ammonium sulphate and calcium super phosphate.
- It is used in the manufacture of pigments, paints, and detergents.
- It is used in the manufacture of storage batteries.
7.24 Write the conditions to maximise the yield of H2SO4 by Contact process.
Ans: Manufacture of sulphuric acid by Contact process involves three steps.
- Burning of ores to form SO2
- Conversion of SO2 to SO3 by the reaction of the former with O2 (V2O5 is used in this process as a catalyst.)
- Absorption of SO3 in H2SO4 to give oleum (H2S2O7)
The key step in this process is the second step. In this step, two moles of gaseous reactants combine to give one mole of gaseous product. Also, this reaction is exothermic.
Thus, in accordance with Le Chatelier’s principle, to obtain the maximum amount of SO3 gas, temperature should be low and pressure should be high.
7.25 Why is Ka2 << Ka1 for H2SO4 in water?
Ans: H2SO4 is a very strong acid in water largely because of its first ionisation to H3O+ and HSO4– The ionisation of HSO4– to H3O+ and SO42- is very very small. That is why, Ka2« Ka1.
7.26 Considering the parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy, compare the oxidising power of F2 and Cl2 .
Ans: The oxidising powers of both the members of halogen family are expressed in terms of their electron accepting tendency and can be compared as their standard reduction potential values.
The oxidising powers of both the members of halogen family are expressed in terms of their electron accepting tendency and can be compared as their standard reduction potential values.
F2 + 2e– → 2F–; E° = 2-87 V, Cl2 + 2e– → 2Cl– ; E° = 1-36 V
Since the E° of fluorine is more than that of chlorine, it is a stronger oxidising agent.
Explanation : Three factors contribute towards the oxidation potentials of both the halogens. These are :
- Bond dissociation enthalpy: Bond dissociation enthalpy of F2 (158 kJ mol-1) is less compared to that of Cl2 (242·6 kJ mol-1).
- Electron gain enthalpy: The negative electron gain enthalpy of F (- 332·6 kJ mol-1) is slightly less than of Cl (-348·5 kJ mol-1).
- Hydration enthalpy: The hydration enthalpy of F- ion (515 kJ mol-1) is much higher than that of Cl- ion (381 kJ mol-1) due to its smaller size.
From the available data, we may conclude that lesser bond dissociation enthalpy and higher hydration enthalpy compensate lower negative electron gain enthalpy of fluorine as compared to chlorine. Consequently, F2 is a more powerful oxidising agent than Cl2.
7.27 Give two examples to show the anomalous behaviour of fluorine.
Ans: Anomalous behaviour of fluorine
- It forms only one oxoacid as compared to other halogens that form a number of oxoacids
- Ionisation enthalpy, electronegativity, and electrode potential of fluorine are much higher than expected.
7.28 Sea is the greatest source of some halogens. Comment.
Ans: Sea water contains chlorides, bromides, and iodides of Na, K, Mg, and Ca. However, it primarily contains NaCl. The deposits of dried up sea beds contain sodium chloride and carnallite, KCl.MgCl2.6H2O. Marine life also contains iodine in their systems. For example, sea weeds contain upto 0.5% iodine as sodium iodide. Thus, sea is the greatest source of halogens.
7.29 Give the reason for bleaching action of Cl2 .
Ans: Chlorine bleaches by oxidation Cl2 + H2O → HCl + HOCl → HCl + [O]
The nascent oxygen reacts with dye to make it colourless.
7.30 Name two poisonous gases which can be prepared from chlorine gas.
Ans: COCl2 (phosgene), CCl3NO2 (tear gas)
7.31 Why is ICl more reactive than I2?
Ans: ICl is more reactive than I2 because I−Cl bond in ICl is weaker than I−I bond in I2
7.32 Why is helium used in diving apparatus?
Ans: A mixture of helium and oxygen does not cause pain due to very low solubility of helium in blood as compared to nitrogen.
7.33 Balance the following equation: XeF6 + H2O → XeO2F2 + HF
Ans: Balanced equation XeF6 + 2 H2O → XeO2F2 + 4 HF
7.34 Why has it been difficult to study the chemistry of radon?
Ans: It is difficult to study the chemistry of radon because it is a radioactive substance having a half-life of only 3.82 days. Also, compounds of radon such as RnF2 have not been isolated. They have only been identified.

