Chapter 4 Chemical Kinetics Class 12 Chemistry Notes for CBSE Students
4.1 For the reaction R → P, the concentration of a reactant changes from 0.03M to 0.02M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Ans: Average rate=

Average rate = 4 x 10-4 mol L-1 min-1 4×10-4
= 60 mol L-1 s-1 = 6.66 x 10-6 mol L-1 s-1
Hence average rate = 4 x 10-4 mol L-1 min-1
Average rate = 6.66 x 10-6 mol L-1 s-1
4.2 In a reaction, 2A → Products, the concentration of A decreases from 0.5 mol L–1 to 0.4 mol L–1 in 10 minutes. Calculate the rate during this interval?
Ans:
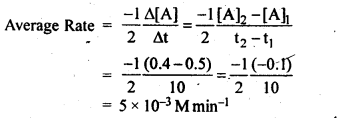
4.3 For a reaction, A + B → Product; the rate law is given by, r = k [ A]1/2 [B]2 . What is the order of the reaction?
Ans: Rate law(r) = k [A] 1/2[B]2
order of reaction = 12+2 =2 1 / 2 or 2.5
4.4 The conversion of molecules X to Y follows second order kinetics. If concentration of X is increased to three times how will it affect the rate of formation of Y?
Ans: The reaction X → Y follows second order kinetics.
Therefore, the rate equation for this reaction will be:
Rate = k[X]2 (1)
Let [X] = a mol L−1, then equation (1) can be written as:
Rate1 = k .(a)2 = ka2
If the concentration of X is increased to three times, then [X] = 3a mol L−1
Now, the rate equation will be:
Rate = k (3a)2
= 9(ka2)
Hence, the rate of formation will increase by 9 times.
4.5 A first order reaction has a rate constant 1.15 × 10-3 s-1. How long will 5 g of this reactant take to reduce to 3 g?
Ans:
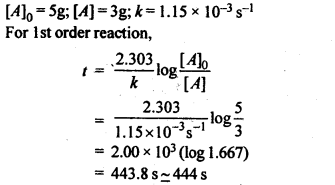
4.6 Time required to decompose SO2Cl2 to half of its initial amount is 60 minutes. If the decomposition is a first order reaction, calculate the rate constant of the reaction.
Ans: We know that for a 1st order reaction, t1/2=0.693/k
It is given that t1/2 = 60 min

4.7 What will be the effect of temperature on rate constant?
Ans: In general, the rate constant for a reaction nearly becomes double with about 10° rise in temperature because of the fact that the effective collisions become almost double. The exact dependence of the reaction rate on temperature is given by Arrhenius equation; k=Ae−Ea/Rt.
Where A is the Arrhenius factor or the frequency factor. It is also called pre exponential factor. It is a constant specific to a particular reaction. R is gas constant and Ea is activation energy measured in joules/mole (J mol-1).
4.8 The rate of the chemical reaction doubles for an increase of 10K in absolute temperature from 298K. Calculate Ea .
Ans:

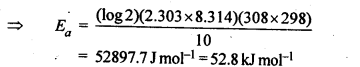
4.9 The activation energy for the reaction 2 HI(g) → H2 + I2 (g) is 209.5 kJ mol–1 at 581K.Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy?
Ans: In the given case: Ea = 209.5 kJ mol−1 = 209500 J mol−1
T = 581 K
R = 8.314 JK−1 mol−1
Now, the fraction of molecules of reactants having energy equal to or greater than
activation energy is given as:
x = e−Ea/RT
⇒ In x=−Ea/RT ⇒ log x=−Ea/2.303RT ⇒ log x=−209500 J mol−1/2.303×8.314 JK−1mol−1×581= −18.8323
==Now, x =Antilog (−18.8323)
= = 1.471×10−19


