Chapter 9 Coordination Compounds Class 12 Chemistry Notes for CBSE Students
9.1 Write the formulas for the following coordination compounds:
1. Tetraamminediaquacobalt(III) chloride
2. Potassium tetracyanidonickelate(II)
3. Tris(ethane–1,2–diamine) chromium(III) chloride
4. Amminebromidochloridonitrito-N-platinate(II)
5. Dichloridobis(ethane–1,2–diamine)platinum(IV) nitrate
6. Iron(III) hexacyanidoferrate(II)
Ans:
- [CO(NH3)4(H2O)2]Cl3
- K2[Ni(CN)4]
- [Cr(en)3]Cl3
- [Pt (NH3) Br Cl (N02)]–
- [PtCl2(en)2](NO3)2
- Fe4[Fe(CN)6]3
9.2 Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6 ]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3 [Fe(CN)6 ]
(iv) K3 [Fe(C2O4 )3 ]
(v) K2 [PdCl4 ]
(vi) [Pt(NH3 )2Cl(NH2CH3 )]Cl
Ans:
- Hexaamminecobalt (III) chloride
- Pentaamminechloridocobalt (III) chloride (HO3)2
- Potassium hexacyanoferrate (III)
- Potassium trioxalatoferrate (III)
- Potassium tetrachloridopalladate (II)
- Diamminechlorido (methylamine) platinum (II) chloride
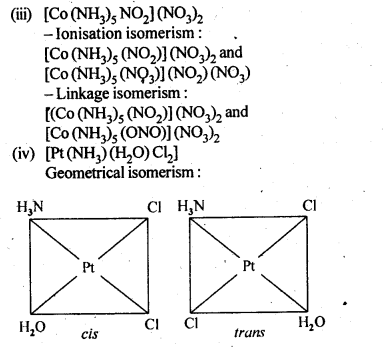
9.3 Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
(i) K[Cr(H2O)2 (C2O4 )2
(ii) [Co(en)3 ]Cl3
(iii) Co(NH3 )5 (NO2 )2
(iv) [Pt(NH3 )(H2O)Cl2 ]
Ans:
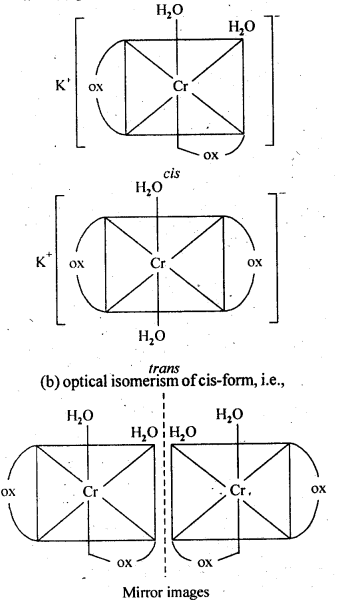
- Both geometrical (cis-, trans-) isomers for K[Cr(H2O)2 (C2O4 )2 can exist. Also, optical isomers for cis-isomer exist.
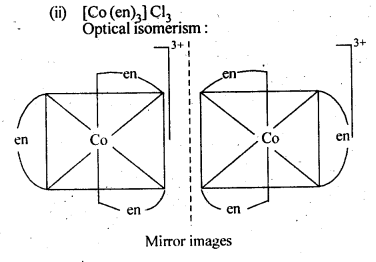
- Two optical isomers for [Co(en)3 ]Cl3 exist.
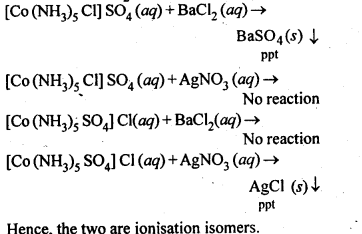
9.4 Give evidence that [Co(NH3 )5Cl]SO4 and [Co(NH3 )5 (SO4 )]Cl are ionisation isomers.
Ans: When ionization isomers are dissolved in water, they ionize to give different ions. These ions then react differently with different reagents to give different products.
9.5 Explain on the basis of valence bond theory that [Ni(CN)4 ] 2– ion with square planar structure is diamagnetic and the [NiCl4 ] 2– ion with tetrahedral geometry is paramagnetic.
Ans: Ni is in the +2 oxidation state i.e., in d8 configuration.
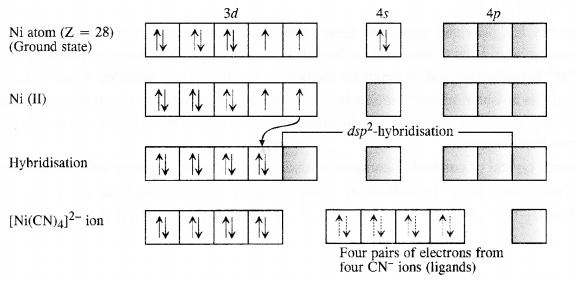
Outer electronic configuration of nickel (Z = 28) in ground state is 3d84s2. Nickel in this complex is in + 2 oxidation state. It achieves + 2 oxidation state by the loss of the two 4s-electrons. The resulting Ni2+ ion has outer electronic configuration of 3d8. Since CN– ion is a strong field, under its attacking influence, two unpaired electrons in the 3d orbitals pair up.
It now undergoes dsp2 hybridization. Since all electrons are paired, it is diamagnetic. In case of [NiCl4] 2−, Cl− ion is a weak field ligand.
Therefore, it does not lead to the pairing of unpaired 3d electrons. Therefore, it undergoes sp3 hybridization.
9.6 [NiCl4 ] 2– is paramagnetic while [Ni(CO)4 ] is diamagnetic though both are tetrahedral. Why?
Ans: Though both [NiCl4] 2− and [Ni(CO)4] are tetrahedral, their magnetic characters are different. This is due to a difference in the nature of ligands. Cl− is a weak field ligand and it does not cause the pairing of unpaired 3d electrons. Hence, [NiCl4] 2− is paramagnetic.

In Ni(CO)4, Ni is in the zero oxidation state i.e., it has a configuration of 3d 8 4s 2
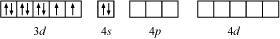
But CO is a strong field ligand. Therefore, it causes the pairing of unpaired 3d electrons. Also, it causes the 4s electrons to shift to the 3d orbital, thereby giving rise to sp3 hybridization. Since no unpaired electrons are present in this case, [Ni(CO)4] is diamagnetic.
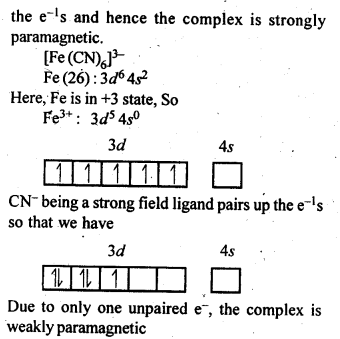
9.7 [Fe(H2O)6 ] 3+ is strongly paramagnetic whereas [Fe(CN)6 ] 3– is weakly paramagnetic. Explain.
Ans: In presence of CN–, (a strong ligand), the 3d electrons pair up leaving only one unpaired electron.
The hybridisation is d2 sp3 forming inner orbital complex. In the presence of H2O, (a weak ligand), 3d electrons do not pair up.
The hybridisation is sp3 d2 forming an outer orbital complex containing five unpaired electrons hence, it is strongly paramagnetic.
9.8 Explain [Co(NH3 )6 ] 3+ is an inner orbital complex whereas [Ni(NH3 )6 ] 2+ is an outer orbital complex.
Ans:
| [Co(NH3)6]3+ | [Ni(NH3)6]2+ | |
| Oxidation state of cobalt = +3 | Oxidation state of Ni = +2 | |
| Electronic configuration of cobalt = d6 | Electronic configuration of nickel = d8 |
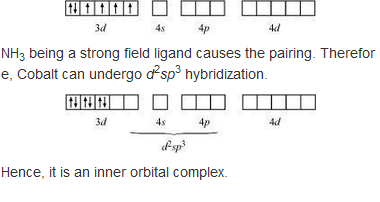
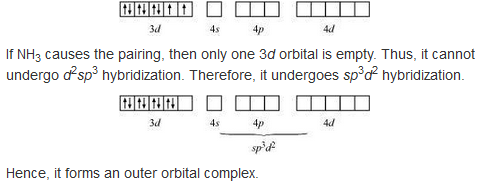
in the presence of NH3, the 3d electrons pair up leaving two d orbitals empty to be involved in d2 sp3 hybridisation forming inner orbital complex in case of [CO(NH3)6]3+.
In Ni(NH3)6]2+, Ni is in +2 oxidation state and has d3 configuration, the hybridisation involved is sp3 d2 forming outer orbital complex.
9.9 Predict the number of unpaired electrons in the square planar [Pt(CN)4 ] 2– ion.
Ans: For square planer shape, the hybridisation is dsp2 . Hence, the unpaired electrons in 5d orbital pair up to make one f-orbital empty for dsp2 hybridisation. Thus there is no unpaired electron.
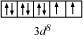
In this complex, Pt is in the +2 state. It forms a square planar structure. This means that it undergoes dsp2 hybridization. Now, the electronic configuration of Pd(+2) is 5d 8.
CN− being a strong field ligand causes the pairing of unpaired electrons. Hence, there are no unpaired electrons in [Pt(CN)4 ] 2–
9.10 The hexaquo manganese(II) ion contains five unpaired electrons, while the hexacyanoion contains o nly one unpaired electron. Explain using Crystal Field Theory.
Ans: Mn(II) has 3d5 electronic configuration. Water is a weak field ligand and therefore ∆0 is small. Therefore, the hexaaqua complex will be high spin complex containing 5 unpaired electrons. On the other hand, CN– is a strong field ligand and therefore, ∆0 is large. Therefore, in its cyano complex, the electrons pair up and have only one unpaired electron.
| [Mn(H2O)6]2+ | [Mn(CN)6]4- |
|---|---|
| Mn is in the +2 oxidation state. | Mn is in the +2 oxidation state |
| The electronic configuration is d5. | The electronic configuration is d5. |
| The crystal field is octahedral. Water is a weak field ligand.Therefore, the arrangement of the electrons in [Mn(H2O)6]2+ is t2g3eg2. | The crystal field is octahedral. Cyanide is a strong field ligand. Therefore, the arrangement of the electrons in [Mn(CN)6]4- is T2g5eg0. |
| Hence, hexaaquo manganese (II) ion has five unpaired electrons | While hexacyano ion has only one unpaired electron. |