Chapter 13 Amines Class 12 Chemistry Notes
13.1 Classify the following amines as primary, secondary or tertiary:
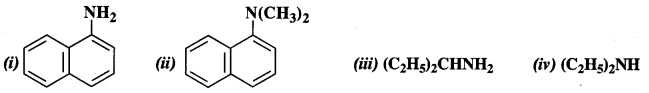
Ans:
(i) Primary
(ii) Tertiary
(iii) Primary
(iv) Secondary
13.2 (i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N.
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
Ans: The structures and their IUPAC names of different isomeric amines corresponding to the molecular formula, C4H11N
(i) CH3-CH2-CH2-CH2-NH2 [Butanamine (10)]
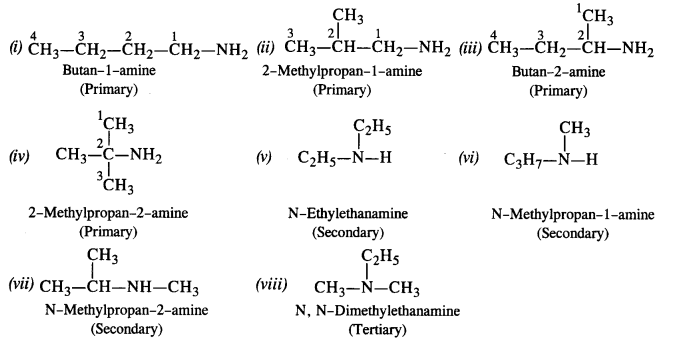
Isomerism exhibited by different amines
- Chain isomers: (i) and (ii) ; (iii) and (iv) ; (i) and (iv)
- Position isomers: (ii) and (iii) ; (ii) and (iv)
- Metamers: (v) and (vi) ; (vii) and (viii)
- Functional isomers: All the three types of amines are the functional isomers of each other
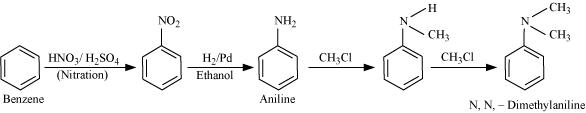
13.3 How will you convert
(i) Benzene into aniline
(ii) Benzene into N, N-dimethylaniline
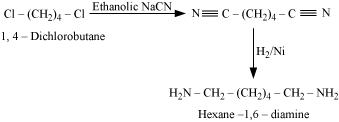
(iii) Cl–(CH2 ) 4 –Cl into hexan-1,6-diamine?
Ans: (i) Nitration of benzene gives nitrobenzene. And now reduce the nitro group by catalytic hydrogenation.
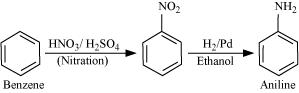
(ii) Nitration of benzene gives nitrobenzene and after catalytic hydrogenation of nitrobenzene, it gives aniline.
(iii) ON reacting the given reactant with ethanolic sodium cyanide, the CN molecules replace both chlorine atom.
13.4 Arrange the following in increasing order of their basic strength:
(i) C2H5NH2 , C6H5NH2 , NH3 , C6H5CH2NH2 and (C2H5 ) 2NH
(ii) C2H5NH2 , (C2H5 ) 2NH, (C2H5 ) 3N, C6H5NH2
(iii) CH3NH2 , (CH3 ) 2NH, (CH3 ) 3N, C6H5NH2 , C6H5CH2NH2
Ans: (i) The order of basicity is C6H5NH2 < NH3 < C6H5CH2NH2 < C2H5NH2 < (C2H5)2NH
The basic nature of amine arises from their ability to donate the lone pair of electrons on N to electrophiles.
The availability of this l.p. depends on two factors :
- Electron donating/withdrawing effect of the alkyl groups attached to the N atom.
- Steric hindrance posed by alkyl groups around N.
(ii) Due to the – R effect of C6H5 group, the electron density on the N atom in C6H5 NH2 is lower than that on the N atom in C2H5NH2. Therefore, the basicity of C6H5NH2 is lower than that of C2H5NH2. Hence, the given compounds can be arranged in the increasing order of their basic strengths as follows:
C6H5NH2 < C2H5NH2 < (C2H5)3N <(C2H5)2NH
(iii) Considering the inductive effect and the steric hindrance of alkyl groups, CH3NH2, (CH3)2NH, and (CH3)3N can be arranged in the increasing order of their basic strengths as
C6H5NH2 < C6H5CH2NH2 < (CH3)3 N < CH3NH2<(CH3)2NH
13.5 Complete the following acid-base reactions and name the products:
(i) CH3CH2CH2NH2 + HCl →
(ii) (C2H5 ) 3N + HCl →
Ans: (i) The above-given reaction is an acid-base reaction. so salt is formed
(ii) (C2H5)3N Triethylamine+ HCI → (C2H5)3N+HCI – Triethylammoniumchloride
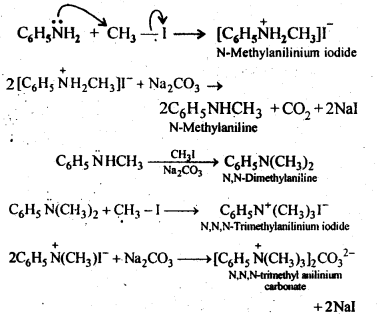
13.6 Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution.
Ans: The methyl iodide reacts with aniline to give N, N-dimethylaniline.
With the excess of methyl iodide in the presence of sodium carbonate solution ( ), N, N-dimethylaniline produces N, N, N-trimethyl anilinium carbonate.
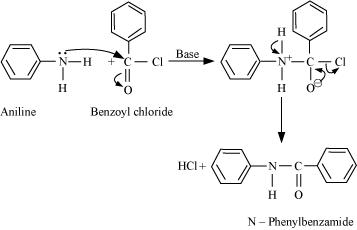
13.7 Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
Ans: When aniline reacts with benzoyl chloride HCl is produced as a by-product and Nphenyl benzamide is produced as a major product. lone pair of N atom attacks the acidic carbon of benzoyl chloride.
The reaction will take place in the presence of aqueous alkali.
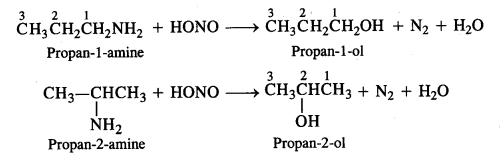
13.8 Write structures of different isomers corresponding to the molecular formula, C3H9N. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
Ans: The structures of different isomers corresponding to the molecular formula, C3H9N are given below:
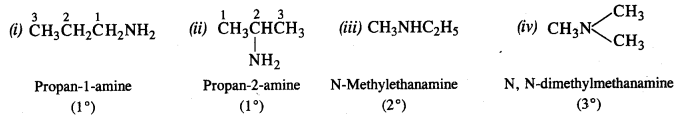
13.9 Convert
(i) 3-Methylaniline into 3-nitrotoluene.
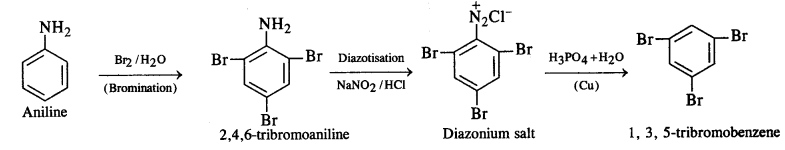
(ii) Aniline into 1,3,5 – tribromobenzene.
Ans: (i) On diazotisation, reaction NH2 will convert to N2CL
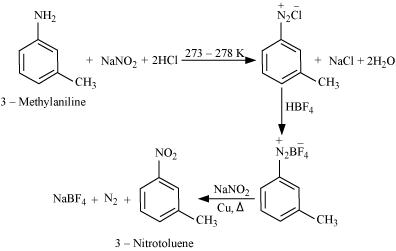
(ii) In the presence of water gives 2,4,6 tribromoaniline, which on further reacting with nitrous acid converts NH2 into N2CL