Chapter 10 Haloalkanes and Haloarenes Class 12 Chemistry Notes for CBSE Students
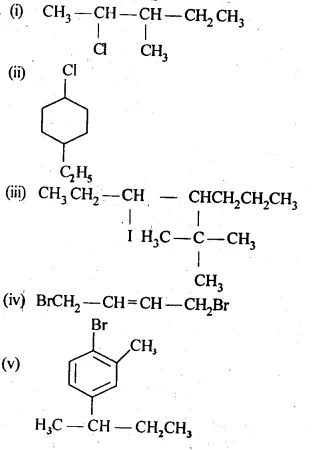
10.1 Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene.
Ans:
10.2 Why is sulphuric acid not used during the reaction of alcohols with KI?
Ans: In the presence of sulphuric acid (H2SO4), KI produces HI
2KI + H2SO4 → 2KHSO4 + 2HI
Since H2SO4 is an oxidizing agent, it oxidizes HI (produced in the reaction to I2).
2HI + H2SO4 → I2 + SO2 + H2O
As a result, the reaction between alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidizing acid such as H3PO4 is used.
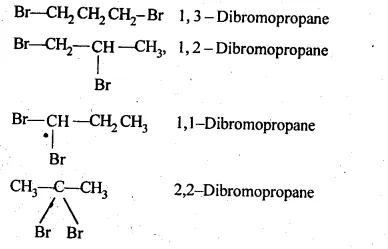
10.3 Write structures of different dihalogen derivatives of propane?
Ans: There are four different dihalogen derivatives of propane.
The structures of these derivatives are shown below.
10.4 Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields
(i) A single monochloride.
(ii) Three isomeric monochlorides.
(iii) Four isomeric monochlorides.
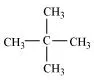
Ans: (i) To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula C5H12. This is because, replacement of any H-atom leads to the formation of the same product. The isomer is neopentane.
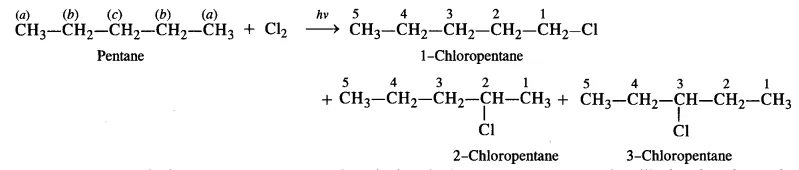
(ii) To have three isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain three different types of H-atoms.
Therefore, the isomer is n-pentane.
It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane.
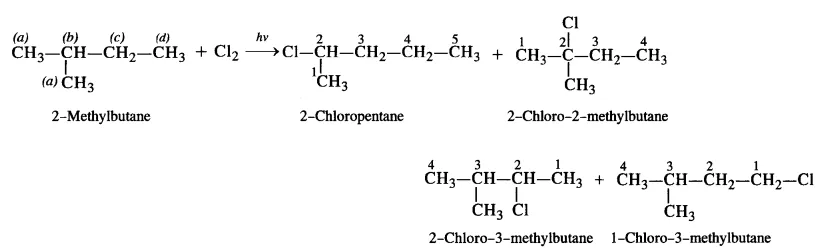
(iii) The branched chain isomer has four types of equivalent hydrogen atoms present. It will give four isomeric monochlorides upon chlorination.
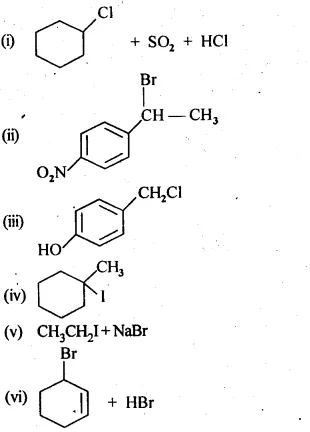
10.5 Draw the structures of major monohalo products in each of the following reactions:
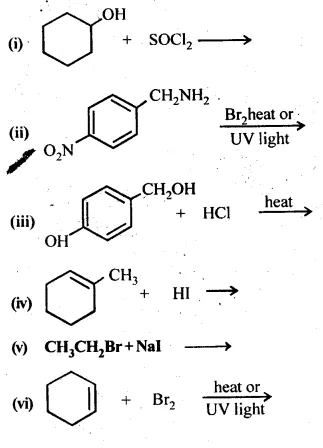
Ans:
10.6 Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane
Ans:
(i) Chloromethane < Bromomethane <Dibromomethane < Bromoform
The reason is:
(a)for same alkyl group, B.Pt increases with size of halogen atom.
(b)B.Pt increases as number of halogen atoms increase.
(ii) Isopropyl chloride < 1 – Chloropropane < 1 – Chlorobutane
Reason
(a)For same halogen, B.Pt. increases as size of alkyl group increases.
(b)B.Pt. decreases as branching increases.
10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an SN 2 mechanism? Explain your answer.
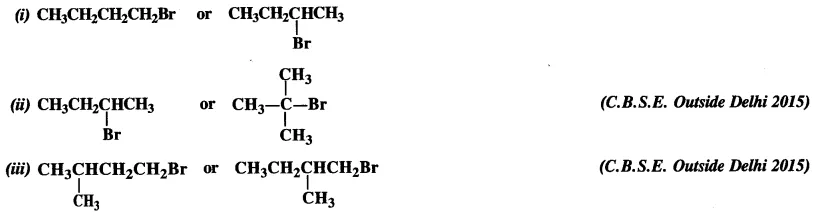
Ans: (i)

2-bromobutane is a 2° alkylhalide whereas 1-bromobutane is a 1° alkyl halide. The approaching of nucleophile is more hindered in 2-bromobutane than in 1-bromobutane. Therefore, 1-bromobutane reacts more rapidly than 2-bromobutane by an SN2 mechanism.
(ii) is a secondary alkyl halide (2°). It is more reactive than the other isomer which is a tertiary alkyl halide (3°). The explanation is the same.
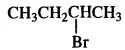
(iii) Here both the isomers are primary alkyl halides (1°). However, the isomer with CH3 group at C2 atom exerts more steric hindrance to the attacking nucleophile at C1 atom as compared to the other isomer in which a CH3 group is attached to C3 atom. It is, therefore, less reactive.
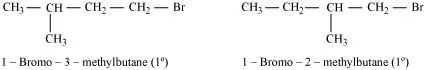
10.8 In the following pairs of halogen compounds, which compound undergoes faster SN 1 reaction?

Ans: The reactivity of a particular halogen compound towards SN¹ reaction depends upon the stability of the carbocation formed as a result of ionisation. This is a slow step and is called rate determining step. The order of relative stabilities of different carbocations is in the order : tertiary > secondary > primary. In the light of this, the order of reactivity in the two cases is explained.
- The isomer (a) is a tertiary alkyl chloride while the other isomer (b) is a secondary alkyl chloride. The isomer (a) is more reactive towards S i reaction since the tertiary carbocation formed in this case is more stable than the secondary carbocation which is likely to be formed in the other case.
- The isomer (a) is a secondary alkyl chloride while the other isomer (b) is primary in nature.
- The secondary alkyl chloride (a) is expected to react faster since the secondary carbocation formed is more stable than the primary carbocation which is likely to be formed in the other case.
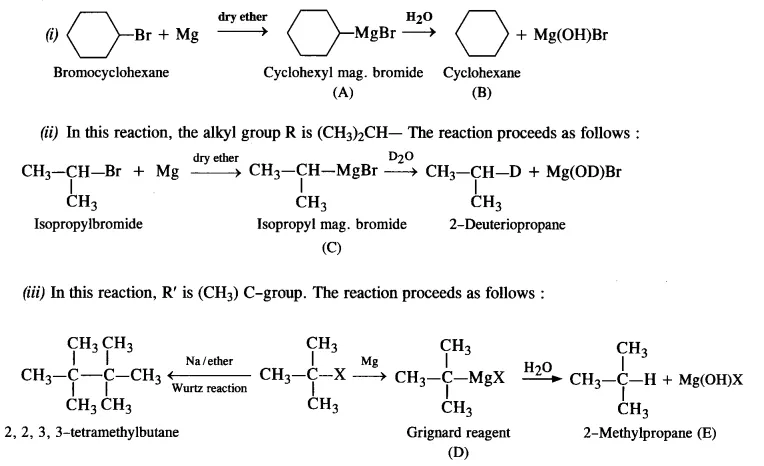
10.9 Identify A, B, C, D, E, R and R1 in the following:
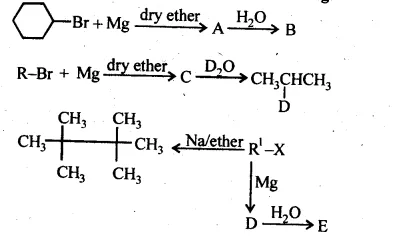
Ans:
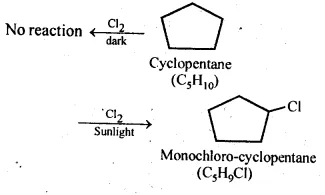
10.10 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans: The hydrocarbon with molecular formula C5H10 can either a cycloalkane or an alkene. Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.